Resource Library

Note: The materials listed on this page might be more current than vaccine administration information in previously published CDC documents, including the 13th edition of Epidemiology and Prevention of Vaccine-Preventable Diseases (the Pink Book). Always follow the most up-to-date guidelines in the Vaccine Storage and Handling Toolkit or more recently dated materials.
Web-based Training Courses
Vaccine Administration e-Learn
A self-paced vaccine administration course that provides comprehensive training using videos, job aids, and other resources.
You Call the Shots
An interactive, web-based immunization training course that includes the latest guidelines and recommendations in vaccine practice.
Videos
Title: Comfort and Restraint Techniques: Infants Younger than 12 Months*
Short Description: This training demonstrates comfort and restraint techniques for infants younger than 12 months. Including parents when vaccines are given can make an infant or child more comfortable. The training will enable you to instruct the parent on how to help the infant or child stay still so you can administer the vaccine(s) safely.
Title: Comfort and Restraint Techniques: Children 12 Through 35 Months of Age*
Short Description: This training demonstrates comfort and restraint techniques for children 12 through 35 months of age. Vaccine injections are a common source of procedural pain and anxiety for children. Including parents when vaccines are given can make an infant or child more comfortable. The training will enable you to instruct the parent on how to help the infant or child stay still so you can administer the vaccine(s) safely.
Title: Comfort and Restraint Techniques: Children 3 Years of Age and Older*
Short Description: This training demonstrates comfort and restraint techniques for children 3 years of age and older. The best position for the patient is based on comfort, age, activity level, administration site, and safety. This training will enable you to instruct the parent on how to help the child stay still so you can administer the vaccine(s) safely.
Title: Assemble a Manufacturer-filled Syringe
Short Description: This training addresses how to assemble a manufacturer-filled syringe, available for a variety of vaccines. CDC recommends that providers only prepare vaccines just prior to administration. Always prepare vaccines in a designated area that is not near any area where potentially contaminated items are placed.
Title: Single-Dose Vial
Short Description: This training addresses how to prepare vaccine from a single-dose vial. A single-dose vial contains one dose and should be administered one time to one patient. CDC recommends that providers only prepare and draw up any vaccine just prior to administration.
Title: Expiration Date
Short Description: This training addresses how to determine when a vaccine or diluent expires—a critical step in vaccine preparation. All vaccines and diluents have an expiration date that indicates the date by which the product must be used. Vaccines and diluents may be used up to and including the expiration date unless the manufacturer indicates otherwise.
Title: Multidose Vial (MDV)
Short Description: This training addresses how to prepare vaccine from a multidose vial (MDV), which contains more than one dose of vaccine. CDC recommends that providers only prepare and draw up any vaccine just prior to administration.
Title: Beyond Use Date (BUD)
Short Description: This training addresses beyond use dates (BUDs) and how to calculate them. Sometimes vaccines must be used before the expiration date—by an earlier date known as the “beyond use date” (BUD). A BUD is calculated based on the date the vial is first entered and the storage information in the vaccine’s package insert. A BUD replaces the original expiration date.
Title: Reconstitute Lyophilized Vaccine
Short Description: This training addresses how to reconstitute a lyophilized (freeze-dried) vaccine. Lyophilized vaccine comes in the form of a powder or pellet and must be mixed with a liquid diluent before the vaccine can be administered. This process is known as “reconstitution.” Diluents vary in volume and ingredients and are specifically designed to mix with their corresponding vaccines.
Title: Rotarix (RV1)
Short Description: This training addresses how to administer RV1 rotavirus vaccine, or Rotarix. RV1 is a live, attenuated vaccine that protects infants from rotavirus infections. It is administered orally as a series of 2 doses. Rotavirus is a contagious virus that can cause gastroenteritis (inflammation of the stomach and intestines), severe, watery diarrhea, vomiting, fever, and abdominal pain. An infant can receive the first dose of RV1 at 2 months of age.
Title: RotaTeq (RV5)
Short Description: This training addresses how to administer RV5 rotavirus vaccine, or RotaTeq. RV5 protects infants from rotavirus infections and is administered orally in a series of 3 doses. Rotavirus is a contagious virus that can cause gastroenteritis (inflammation of the stomach and intestines); severe, watery diarrhea; vomiting; fever; and abdominal pain. An infant can receive the first dose of RV5 at 2 months of age.
Title: Live, Attenuated Influenza Vaccine (LAIV)
Short Description: This training addresses how to administer FluMist, the live, attenuated influenza vaccine (LAIV). FluMist is the only vaccine administered by the intranasal route. Influenza (flu) is a contagious respiratory illness caused by influenza viruses. It can cause mild to severe illness and result in serious outcomes such as hospitalization or death. The annual recommendation for influenza vaccination for children and adults means that health care providers should stay up to date in their knowledge of influenza vaccination recommendations. The best way to prevent the flu is to get vaccinated each year.
Title: Subcutaneous (SC or Subcut) Injection: Administration
Short Description: This training addresses how to administer a subcutaneous (SC or subcut) injection. Injections are commonly used in health care settings to administer vaccines for disease prevention. A needle is used to inject the vaccine into the tissue layer between the skin and the muscle. Safe injection practices minimize risk of injuries, infections, and non-infectious adverse events for both patients and providers. Health care providers are always advised to observe patients for 15 minutes after vaccination.
Title: Subcutaneous (SC or Subcut) Injection: Supplies
Short Description: This training addresses how to select the equipment needed to prepare for a subcutaneous (SC or subcut) injection. Aseptic technique must be used to protect vaccines, injection equipment, and supplies from microbial contamination. Safe and sterile injection practices minimize risk of injuries, infections, and non-infectious adverse events for both patients and providers.
Title: Subcutaneous (SC or Subcut) Injection: Sites
Short Description: This training helps providers identify subcutaneous (SC or subcut) injection sites. A needle is used to inject the vaccine into the tissue layer between the skin and the muscle. The appropriate site for a subcutaneous injection for those under 12 months of age is the fatty tissue over the anterolateral thigh. The fatty tissue over the triceps area of the upper arm is preferred for those older than 12 months of age. Safe injection practices minimize risk of injuries, infections, and non-infectious adverse events for both patients and providers. Health care providers are always advised to observe patients for 15 minutes after vaccination.
Title: Intramuscular (IM) Injection: Supplies (Children Birth through 18 Years of Age)
Short Description: This training addresses how to select the equipment needed to prepare an intramuscular (IM) injection for children from birth through 18 years of age. A supply of needles of the appropriate lengths should be available. Aseptic technique must be used to protect supplies from microbial contamination. Safe injection practices minimize risk of injuries, infections, and non-infectious adverse events for both patients and providers. Health care providers are always advised to observe patients for 15 minutes after vaccination.
Title: Intramuscular (IM) Injection: Supplies (Adults 19 Years of Age and Older)
Short Description: This training addresses how to select the equipment needed to prepare an intramuscular (IM) injection for adults 19 years of age and older. A supply of needles of the appropriate lengths should be available. Aseptic technique must be used to protect supplies from microbial contamination. Safe injection practices minimize risk of injuries, infections, and non-infectious adverse events for both patients and providers. Health care providers are always advised to observe patients for 15 minutes after vaccination.
Title: Intramuscular (IM) Injection: Sites
Short Description: This training helps providers identify intramuscular (IM) injection sites. A needle is used to inject the vaccine into the muscle. The appropriate site for an intramuscular injection for those under 2 years of age is the vastus lateralis muscle. The deltoid muscle over the triceps area of the upper arm is preferred for persons 3 years of age and older. Safe injection practices minimize risk of injuries, infections, and non-infectious adverse events for both patients and providers. Health care providers are always advised to observe patients for 15 minutes after vaccination.
Title: Fluzone Intradermal
Short Description: This training addresses how to administer Fluzone Intradermal inactivated influenza vaccine to adults 18 to 64 years of age. Influenza (flu) is a contagious respiratory illness caused by influenza viruses. It can cause mild to severe illness and result in serious outcomes such as hospitalization or death. The annual recommendation for influenza vaccination for children and adults means that health care providers should be up to date in their knowledge of influenza vaccination recommendations. The best way to prevent the flu is to get vaccinated each year.
Title: Documentation of Vaccines After Administration
Short Description: This training addresses how to accurately document vaccinations after administration as required by the National Childhood Vaccine Injury Act (NCVIA). Vaccine doses should be documented in both a medical record and immunization information system (IIS). Proper documentation using an IIS can help consolidate vaccination records for patients, lessen the possibility that patients will receive unnecessary doses of vaccines, and provide data for calculating a practice’s vaccination coverage. Health care providers should also give patients, parents, and/or guardians a personal vaccination record that includes the names and dates of vaccines administered.
Job Aids
-
Skills Checklist for Immunization [2 pages]
A self-assessment tool for health care personnel who administer vaccines. -
Suggestions to Improve Your Immunization Services [3 pages]
Tips for improving a clinic’s efficiency in administering vaccines and improving immunization rates and a checklist to help implement and reinforce these suggestions. - At-A-Glance Resource Guide: Vaccine Administration and Storage and Handling [1 page]
- Vaccine Manufacturer/Distributor Contact Information [2 pages] (Updated June 2016)
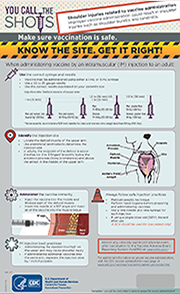
Infographic
This infographic promotes safe vaccination practices by educating and reminding providers about proper influenza (flu) vaccine administration technique.
References
- General Best Practice Guidelines for Immunization: Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP)
-
Immunization Action Coalition’s print materials for health care professionals providing vaccines
Vaccine storage, handling, and administration, documentation of vaccinations, supplies checklist, vaccine questions and answers, etc. - “Vaccine Administration” chapter, Epidemiology and Prevention of Vaccine-Preventable Diseases (the Pink Book)—includes pictures of vaccine administration sites
- Food and Drug Administration (FDA) Product Approval: Vaccine Index—includes vaccine manufacturers’ package inserts
-
Standing Orders for Administering Vaccines
An overview for health care professionals on the use of standing orders for vaccination and steps for implementation. - Ask the Experts: Administering Vaccines
- Vaccines For Children (VFC) Program: CDC Vaccine Price List
- Travel Health (including vaccines)
Resources
Web Button

- Page last reviewed: April 14, 2017
- Page last updated: September 21, 2017
- Content source:


 ShareCompartir
ShareCompartir