Negative Staining Electron Microscope Protocol for Rash Illness
Introduction
Electron microscopic (EM) visualization of negatively stained poxvirus virions was a valuable technique for confirming poxvirus infections during the smallpox eradication campaign. Historically, negative stain EM successfully detected poxvirus particles in approximately 95% of clinical specimens from patients with orthopoxvirus infections such as variola (smallpox)/monkeypox, and approximately 65% from patients with vaccinia (smallpox vaccine). In the event of a deliberate release of smallpox virus and subsequent human disease, or in generalized vaccinia infections resulting from vaccination, negatively stained preparations derived from lesions or scab material would again provide a valuable method for assisting in poxvirus diagnosis and/or ruling out other causes of rash illness. However, EM visualization of virions compatible with a poxvirus, by itself, would not constitute proof of a smallpox infection because different poxviruses, such as variola, vaccinia, monkeypox, and molluscum (molluscipoxvirus) viruses, are morphologically indistinguishable.
While EM laboratories will be integral members of the biopreparedness team, several issues should be considered before an EM laboratory agrees to process specimens from patients with suspect poxvirus infection. First to consider is the diagnostic capabilities of the laboratory personnel, who should have experience with preparing negative stain EM grids, and, more importantly, must have experience with analyzing negative stain EM preparations to identify virus morphology, and to differentiate these from look-alikes and artifacts. Second, EM personnel who will handle specimens need to have been recently vaccinated, or have no contraindications to post-exposure vaccination. Lastly, the EM laboratory will need to have access to a BSL-3 containment facility, or a BSL-2 facility that can use BSL-3 precautions.
EM laboratories involved in negative stain viral diagnostics are encouraged to participate in the External Quality Assessment program administered by the Robert Koch Institut in Berlin. The training is called “EQA-EMV (External Quality Assurance Scheme in Electron Microscopy Virus Diagnostics) – Ring test electron microscopic virus diagnostics.”
Reporting and Appropriate Action
- Pre-event, clinical specimens with high levels of suspicion for presence of variola virus, as described by the Acute, Generalized Vesicular or Pustular Rash Illness Protocol, should be immediately forwarded to CDC for specialized diagnostic evaluation. Several tests to confirm or rule-out smallpox infection will be performed at CDC, given that a positive result for smallpox would precipitate an immediate and extensive public health response.
- Details are available at CDC’s Smallpox homepage.
Materials
Acceptable specimens, from lesions
- Vesicular fluid on EM grid
- Vesicular fluid as smears on glass slides
- Crusts and tissue biopsies
- Swabs
- Vesicular fluid in tuberculin syringe, or other collective device
These collection devices should be shipped inside a sealed plastic container to avoid spillage. Safety precautions are required when using needle and syringe. Consult your local safety officer.
Reagents
- Phosphotungstic acid (see Negative Stain Reagents section for recipe)
- Uranyl acetate (see Negative Stain Reagents section for recipe)
- Sterile deionized water (dH2O)
- 1% Alcian Blue (Sigma Aldrich)
- 2% EM grade paraformaldehyde (Electron Microscopy Sciences) buffered with phosphate buffered saline (PBS, pH 7.3)
- Freshly made 1:10 dilution of commercial bleach (sodium hypochlorite)
Supplies
- Formvar-carbon-coated 400 mesh copper grids (Electron Microscopy Sciences) (On these grids it is the shiny side that is coated with plastic.)
- Grid storage box (Electron Microscopy Sciences)
- Parafilm
- 1 cc syringe (for step II.B.9.)
- Syringe filters (for step II.B.9.)
- Pestle and Grinder Tube set (Fisher Scientific)
- Swab Extraction Tube System (Roche Applied Science)
- Microcentrifuge tubes with O-ring
- Gauze pad, soaked in freshly-made 1:10 solution of commercial bleach
- Optional, for alternate swab preparation (see step I.D.2.): Conical centrifuge tubes, plastic, 15 mL; Syringe, plastic, 3 cc; Wooden applicator stick
- If necessary, for UV irradiation and bleach inactivation (see step III.A.): Petri dishes, plastic, 60 x 15 mm and 100 x 15 mm; Filter paper, round, 55 mm.
Equipment
- Transmission electron microscope
- Tweezers, EM grade (examples include: Ted Pella, Inc. and Electron Microscopy Sciences)
- Microcentrifuge (see step I.C.3.)
- Tabletop centrifuge (see step I.D.2.(f))
- Optional, for virus concentration (see step I.F.): Airfuge (Beckman Instruments)
- Optional, for alternate grid preparation (see step II.A.2.): Glow discharge unit and vacuum pump (Ted Pella, Inc.). Alternately, a glow discharge unit may be constructed in the laboratory, following the design described by Aebi and Pollard (see References).
- If necessary, for UV irradiation and bleach inactivation (see step III.A.): E-Series Germicidal Ultraviolet Lamps, lamp stand, UV meter, UV goggles (Spectroline)
Materials Sources
Beckman Instruments Tel: (800) 742-2345
Electron Microscopy Sciences Tel: (800) 523-5874
Fisher Scientific Tel: (800) 766-7000
Roche Applied Science Tel: (800) 262-1640
Sigma-Aldrich Tel: (800) 325-3010
Spectroline Tel: (800) 274-8888
Ted Pella, Inc. Tel: (800) 237-3526
Disclaimer:
Names of vendors or manufacturers are provided as examples of suitable product sources; inclusion does not imply endorsement by the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Negative Staining Procedure
Negative Stain Reagents
- 2% Phosphotungstic Acid (PTA):
- 2 g phosphotungstic acid in 100 mL dH20
- pH to 7.0 with KOH
- Store at 2 to 8°C
- 0.5% Uranyl Acetate (UA):
- 0.5 g uranyl acetate in 100 mL dH20
- Let stand overnight
- Store at 2 to 8°C, in the dark
- Specimen Preparation
All unfixed material from high risk specimens should be manipulated in the BSL-3.
Note: When possible, prepare at least 2 grids per specimen. If material on EM grid is too dense, dilute the specimen with dH2O and make new grids.- Vesicular fluid on EM grids previously prepared by the direct touch method:
- Proceed to step II.B.9.
- Vesicular fluid as smears on glass slides:
- Add 1-2 drops sterile dH20.
- Scratch dry material to resuspend.
- Make EM grids directly off this material (see step II.)
- Transfer remaining liquid to microfuge tube for storage/further testing.
- Crusts and tissue biopsy (use pestle and grinder tube):
- Place crust in tissue grinder tube and add 1 mL sterile dH20.
- Grind to produce an opalescent suspension.
- Centrifuge at 1,000 x g for 5 minutes.
- Use supernatant as the specimen for step II.
- Swabs
- Either follow directions for the Swab Extraction Tube System (Roche)
- Or follow the alternate procedures (below):
- Place swab in 15 mL conical tube containing approximately 0.3 mL of sterile dH20.
- Soak for 10 to 15 minutes.
- With a wooden applicator stick, scrape any remaining specimen off the cotton swab directly into the dH20.
- Temporarily remove swab from centrifuge tube. Place the barrel only of a 3 cc syringe into the 15 mL conical tube, then place swab into syringe barrel. Break off stick. Screw on cap to prevent aerosolization.
- Place conical tube into a centrifuge canister in an aerosol-barrier rotor.
- Centrifuge at 2,000 x g for 20 minutes.
- Place entire centrifuge canister back into BSC. Remove conical tube from canister.
- Remove and discard swab and syringe barrel into a discarded bin containing a 1:10 dilution of commercial bleach.
- Resuspend any sediment. Use the resulting liquid as the specimen for step II.
- Vesicular fluid in collective devices (e.g., syringe, capillary tube, etc.)
- Expel fluid into microcentrifuge tube.
- Place two 2 to 5 μl drops of specimen onto sheet of Parafilm. (Save any remaining fluid for additional testing.)
- Dilute the 2nd drop by adding an equal amount of dH20 and mixing.
- Proceed to step II.
- Keep remaining specimen in microcentrifuge tube for storage/further testing.
- Virus concentration (optional):
- If available, an airfuge may be used to concentrate the virus in specimens from step I.B.3. (vesicular fluid as smears on glass slides), step I.C.4. (crusts and tissue biopsies), and step I.D.1. Or I.D.2.(i). (swabs).
- Spin specimens at 30 lb/in2 for 30 min., decant supernatant into a discard pan containing freshly diluted bleach solution, resuspend pellet in 10 to 20 μl of dH20 and use liquid as the specimen for step II.
- Vesicular fluid on EM grids previously prepared by the direct touch method:
- EM Grid Processing by the Drop-to-Drop Method
Make at least 2 specimen grids whenever possible.- Enhance hydrophilicity of EM grids:
- Place plastic/carbon-coated grids (plastic side down) on a drop of 1% Alcian Blue for 5 minutes, then rinse with 3 drops of dH2O.
- Alternatively, if available, use glow discharge treatment on grids just prior to applying grid to specimen drop.
- Drop-to-Drop Method (see Fig. 1):
- Place 5 μl of liquid specimen onto a sheet of parafilm.
- NOTE: to avoid cross-contamination, use different tweezers for each specimen.
- Place plastic/carbon-coated 400-mesh copper grid (plastic-side down) on drop and let absorb for 10 minutes.
- Wick away excess fluid with filter paper.
- Place grid within a large drop of buffered 2% paraformaldehyde for 5 minutes.
- Wick away excess fluid with filter paper.
- Rinse in 2 drops of dH2O.
- Wick away excess fluid with filter paper.
- Stain.
- Stains can be stored in 1 cc syringes equipped with syringe filters. Cover the syringe with 0.5% UA in foil to exclude light.
- Place grid (plastic-side down) on drop of filtered 2% PTA, pH 7.0, and let stain for approximately 30 seconds.
- If 2nd specimen grid is available, stain with filtered 0.5% UA for approximately 30 seconds.
- Wick away excess fluid with filter paper.
- If proceeding to step III.A., place grids into the bottom of a 60 x 15 mm plastic petri dish containing a round filter paper. Otherwise, proceed to step III.B.
- Enhance hydrophilicity of EM grids:
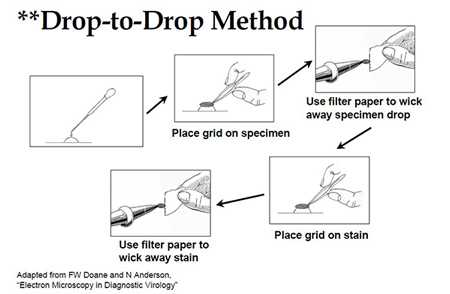
Figure 1: Drop-to-Drop Method illustration outlined in section II.B, for previously fixed specimens.
Adapted from FW Doane and N Anderson, Electron Microscopy in Diagnostic Virology.
- Inactivation
Note: Inactivate grids within the Biological Safety Cabinet.- UV irradiation and bleach inactivation (see Fig. 2)
Note: These steps, using UV irradiation and bleach, are only necessary for specimens that have been designated as a high risk for variola virus. (Please refer to Diagnosis & Evaluation for more information about evaluating specimen risk.) They are used to ensure the inactivation of any virus particles, including those on the filter paper or on the outside of the petri dish.- Add a freshly made 1:10 dilution of commercial bleach to a 100 x 15 mm petri dish, just enough to cover the bottom of the dish (approximately 50 mL).
- Place bottom of specimen petri dish (with grids) within the larger dish.
- Place under UV light (254 nm wavelength) and irradiate 10 minutes.
- Turn grids over and irradiate an additional 10 minutes.
- UV irradiation and bleach inactivation (see Fig. 2)

Figure 2: UV Irradiation and Bleach Inactivation (see step III.A.). Image of equipment described in Step III.A used to inactivate specimens using UV irradiation and bleach inactivation.
-
- Using clean tweezers, place grids into grid storage box. Carefully record which slot is used for each patient specimen. All used EM tweezers should be decontaminated with gauze saturated with a 1:10 bleach solution.
Interpretation of Results
Poxviruses, excluding parapoxviruses
The virions measure approximately 225 X 300 nm, and appear rectangular or brick-shaped when viewed lengthwise and circular or ovoid when viewed on end. Depending on penetration of the stain, two forms may be seen. In the “M” (or “mulberry”) form, the surface is covered with short, whorled filaments, and a circular depression is sometimes seen in the center of the virion. In particles penetrated by stain, the “C” or (“capsular”) form, surface filaments are not visible; instead, the virion consists of a sharply defined, dense core surrounded by several laminated zones of differing densities. In addition, enveloped particles are sometimes found.
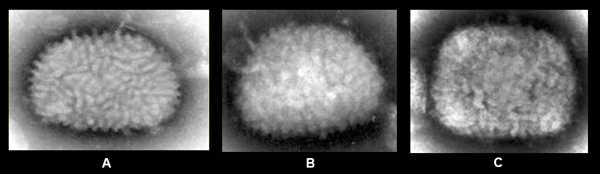
Figure 3: EM of vaccinia virus from tissue culture (A). EM of vaccinia virus from clinical specimen (B). EM of monkeypox virus from clinical specimen (C). Note that in clinical specimens the morphology may be less distinct than in tissue culture specimens.
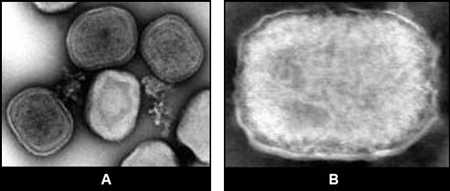
Figure 4: EM of fowlpox virus, tissue culture specimen, showing 3 virions with “C” form (A). EM of tanapox virus, clinical specimen (enveloped virion) (B).
Parapoxviruses (e.g., Orf)
Parapoxvirus particles appear more ovoid than other poxviruses, and the surface filaments have a spiral or criss‑cross arrangement. Particles measure approximately 150 X 200 nm.
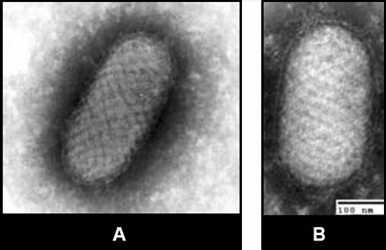
Figure 5 EM of parapoxvirus (Orf virus) from tissue culture (A) and clinical specimen (B). Electron microscopy images of parapox virus from tissue culture and clinical specimen.
Herpesviruses (e.g., varicella zoster virus, herpes simplex viruses type 1 and type 2)
The naked nucleocapsid, measuring approximately 100 nm in diameter, is composed of an icosahedron formed by hollow capsomers. Stain-penetrated nucleocapsids may have the appearance of a hexagon rimmed by the hollow capsomers. Enveloped virions may be identified when the stain penetrates the viral envelope and outlines the nucleocapsid.
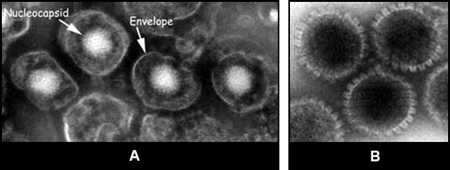
Figure 6:Two images of herpesvirus particles from tissue culture. Enveloped virions (A). Naked nucleocapsids, rimmed by hollow capsomers (B).
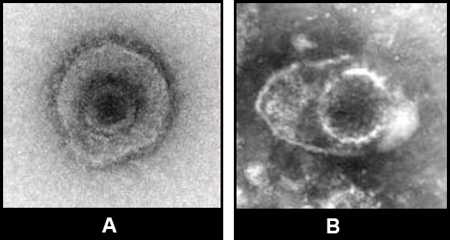
Figure 7: Two images of herpesvirus from clinical specimens. Herpesvirus from clinical specimens. Note that in clinical specimens the morphology may be less distinct than in tissue culture specimens.
Note that in clinical specimens the morphology may be less distinct than in tissue culture specimens.
Melanosomes
Care must be taken to distinguish between poxvirus particles and this look-alike structure found in normal skin. Melanosomes are found in skin epidermis and hair bulbs and measure approximately 370 nm in diameter and 0.7 to 1.15 µm in length.
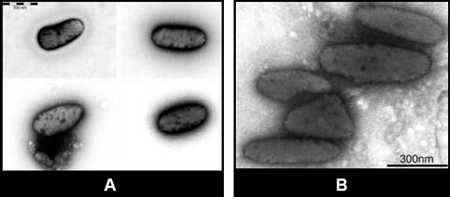
Figure 8: Two EM images of melanosomes.
References
Abei U and Pollard TD. A glow discharge unit to render electron microscopic grids and other surfaces hydrophilic. Journal of Electron Microscopy Techniques. 1987;7:29-33.
Centers for Disease Control and Prevention. Smallpox.
Gelderblom HR and Hazelton PR. Specimen collection for electron microscopy. Emerging Infectious Diseases. 2000;6(4):433-434.
Long GW, Nobel J, Murphy FA, Herrmann KL, and Lourie B. Experience with electron microscopy in the differential diagnosis of smallpox. Applied Microbiology. 1970;20(3):497-504.
Miller SE. Bioterrorism and electron microscopic differentiation of poxviruses from herpesviruses: Dos and Don’ts. Ultrastructural Pathology. 2003;27:133-140.
Nakano JH. Poxviruses. In Lennette EH and Schmidt NJ, editors. Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections, 5th ed. Washington, DC: American Public Health Association. 1979;p.257-308.
- Page last reviewed: December 1, 2016
- Page last updated: December 1, 2016
- Content source:


 ShareCompartir
ShareCompartir