Chart 3: Laboratory Testing for Suspect Smallpox Vaccine Adverse Event (Vaccinia)
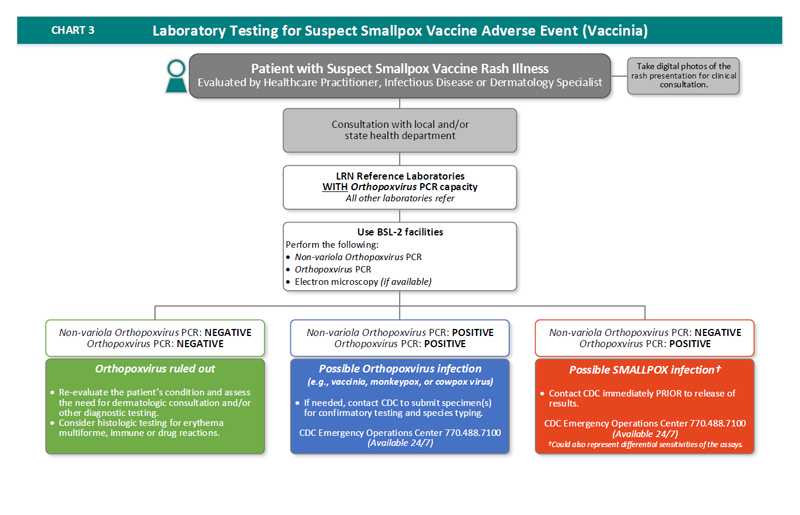
- A patient presents with suspect smallpox vaccine rash illness and is evaluated by healthcare practitioner, infectious disease or dermatology specialist
- Take digital photos of the rash presentation for clinical consultation
- Consult with local and/or state health department
- Laboratory testing occurs in a LRN Reference Laboratory with Orthopoxvirus PCR capacity. All other laboratories refer.
- LRN Reference Laboratory with Orthopoxvirus PCR capabilities must use BSL-2 facilities to perform the following tests:
- Non-variola Orthopoxvirus PCR
- Orthopoxvirus PCR
- Electron microscopy (if available)
- If the Non-variola Orthopoxvirus PCR is negative and the Orthopoxvirus PCR is negative, Orthopoxvirus is ruled out. The risk is low.
- Re-evaluate the patient’s condition and assess the need for dermatologic consultation and/or other diagnostic testing.
- Consider histologic testing for erythema multiforme, immune or drug reactions.
- If the Non-variola Orthopoxvirus PCR is positive and the Orthopoxvirus PCR is positive, the patient has a possible Orthopoxvirus infection other than variola virus (e.g., vaccinia, monkeypox, or cowpox virus).
- If needed, contact CDC to submit specimen(s) for confirmatory testing and species typing. The CDC Emergency Operations Center is available 24/7 at 770-488-7100.
- If the Non-variola Orthopoxvirus PCR is negative and the Orthopoxvirus PCR is positive, the patient has a possible smallpox infection. The results could also represent differential sensitives of the assays.
- Contact CDC immediately PRIOR to release of results. The CDC Emergency Operations Center is available 24/7 at 770-488-7100.
- Page last reviewed: December 1, 2016
- Page last updated: December 1, 2016
- Content source:


 ShareCompartir
ShareCompartir