Chart 2: Laboratory Testing for Acute, Generalized Vesicular or Pustular Rash Illness
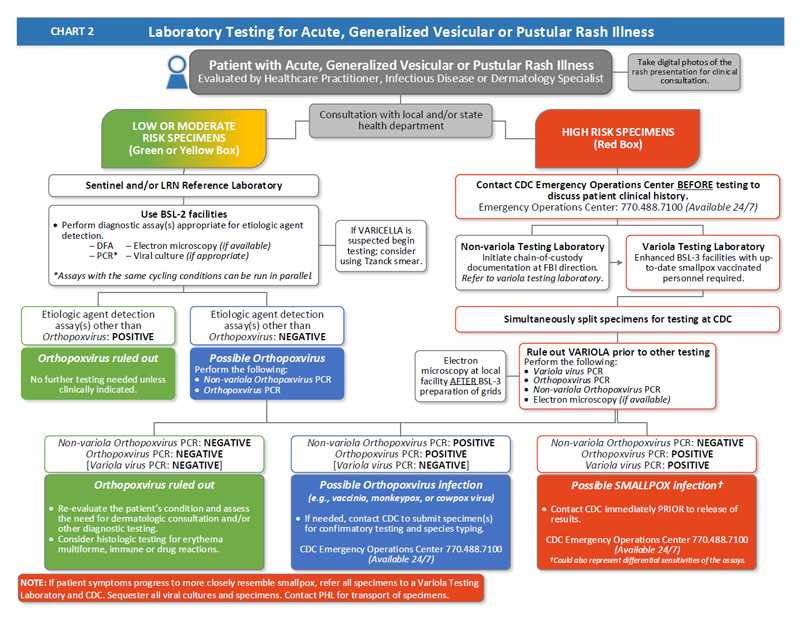
- A patient presents with an acute, generalized vesicular or pustular rash illness and is evaluated by a healthcare practitioner, infectious disease, or dermatology specialist.
- Take digital photos of the rash presentation for clinical consultation.
- Obtain consultation with local and/or state public health department.
- If patient evaluation determines the patient has a low or moderate risk of smallpox, the specimens for testing will also be low or moderate risk and will be tested by a sentinel and/or an LRN Reference Laboratory.
- Use BSL-2 facilities to perform diagnostic assay(s) appropriate for etiologic agent detection. Options are:
- DFA
- PCR (assays with the same cycling conditions can run in parallel)
- Electron microscopy (if available)
- Viral culture (if appropriate)
- If varicella is suspected begin testing; consider using Tzanck smear.
- If etiologic agent detection assay(s) other than Orthopoxvirus are positive:
- Orthopoxvirus is ruled out. No further testing is needed unless clinically indicated. The patient has a low risk of smallpox.
- If etiologic agent detection assay(s) other than Orthopoxvirus are negative:
- Orthopoxvirus is possible.
- Perform the following tests:
- Non-variola Orthopoxvirus PCR
- Orthopoxvirus PCR
- If the Non-variola Orthopoxvirus PCR and Orthopoxvirus PCR are both negative, Orthopoxvirus is ruled out.
- Re‑evaluate the patient’s condition and assess the need for dermatologic consultation and/or other diagnostic testing.
- Consider histologic testing for erythema multiforme, immune or drug reactions.
- If the Non-variola Orthopoxvirus PCR and the Orthopoxvirus PCR are both positive:
- Orthopoxvirus infection other than variola virus (e.g., vaccinia, monkeypox, or cowpox virus) is possible.
- If needed, contact CDC to submit specimen(s) for confirmatory testing and species typing. The CDC Emergency Operations Center is available 24/7 at 770-488-7100.
- Use BSL-2 facilities to perform diagnostic assay(s) appropriate for etiologic agent detection. Options are:
- If patient evaluation determines the patient has a HIGH risk of smallpox:
- Contact CDC Emergency Operations Center at 770-488-7100 before testing to discuss patient clinical history.
- If specimens are at a non-variola testing laboratory:
- Initiate chain-of-custody at FBI direction. Refer to variola testing laboratory.
- If specimens are at a variola testing laboratory:
- Use enhanced BSL-3 facilities. It is required that testing personnel be up‑to‑date with their smallpox vaccination.
- Simultaneously split specimens for testing at CDC.
- Rule out variola prior to other testing. Perform the following:
- Variola virus PCR
- Orthopoxvirus PCR
- Non-variola Orthopoxvirus PCR
- Electron microscopy (if available). Perform electron microscopy at local facility AFTER BSL-3 preparation of grids.
- If the Non-orthopoxvirus PCR is negative, the Orthopoxvirus PCR is negative, and Variola virus PCR is negative:
- Orthopoxvirus is ruled out.
- Re‑evaluate the patient’s condition and assess the need for dermatologic consultation and/or other diagnostic testing.
- Consider histologic testing for erythema multiforme, immune or drug reactions.
- If the Non-orthopoxvirus PCR is positive, the Orthopoxvirus PCR is positive, and Variola virus PCR is negative:
- Orthopoxvirus infection other than variola virus (e.g., vaccinia, monkeypox, or cowpox virus) is possible.
- If needed, contact CDC to submit specimen(s) for confirmatory testing and species typing. The CDC Emergency Operations Center is available 24/7 at 770-488-7100.
- If the Non-orthopoxvirus PCR is negative, the Orthopoxvirus PCR is positive, and Variola virus PCR is negative:
- Smallpox infection is possible (though this could also represent different sensitivities of the assays)
- Contact CDC immediately PRIOR to release of results. Call CDC Emergency Operations Center at 770-488-7100 (available 24/7)
NOTE: If patient symptoms progress to more closely resemble smallpox, refer all specimens to a Variola Testing Laboratory and CDC. Sequester all viral cultures and specimens. Contact PHL for transport of specimens.
- Page last reviewed: December 1, 2016
- Page last updated: December 1, 2016
- Content source:


 ShareCompartir
ShareCompartir