Diagnosis Confirmation
Clinicians commonly use several types of laboratory tests to diagnose Bordetella pertussis. Scientists consider culture the gold standard because it is the only 100% specific method for identification. Other tests that can be performed include polymerase chain reaction (PCR) and serology.
Culture
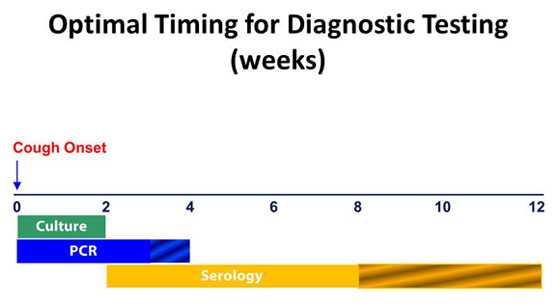
Since culture has excellent specificity, it is particularly useful for confirming pertussis diagnosis when you suspect an outbreak. Many other respiratory pathogens have similar clinical symptoms to pertussis and co-infections do occur. Furthermore, obtaining isolates from culture allows for strain identification and antimicrobial resistance testing. Identifying which strains of B. pertussis are causing disease is of public health importance. It is best for you to obtain a culture from nasopharyngeal (NP) specimens collected during the first 2 weeks of cough. This is when viable bacteria are still present in the nasopharynx. After the first 2 weeks, sensitivity decreases and the risk of false-negatives increases.
PCR
Key Resource
PCR is a rapid test and has excellent sensitivity. However, PCR tests vary in specificity. Therefore, you should obtain culture confirmation of pertussis for at least one suspicious case any time there is suspicion of a pertussis outbreak. You should interpret results along with the clinical symptoms and epidemiological information. You should test with PCR from NP specimens taken at 0 to 3 weeks following cough onset. PCR may also provide accurate results for up to 4 weeks. After the fourth week of cough, the amount of bacterial DNA in the nasopharynx rapidly diminishes, which increases the risk of obtaining falsely-negative results. PCR assay protocols that include multiple target sequences allow for speciation among Bordetella species. The high sensitivity of PCR increases the risk of false-positivity, but following some simple best practices can reduce the risk of obtaining inaccurate results.
Serology
CDC and FDA have developed a serologic assay that has been extremely useful for confirming diagnosis, especially during suspected outbreaks. Many state public health labs have included this assay as part of their testing regimen for pertussis. Commercially, there are several different serologic tests used in the United States with unproven or unknown clinical accuracy. CDC is actively engaged in better understanding the usefulness of these commercially available assays. CDC will update this website as more is learned about these commercial assays. Generally, serologic tests are more useful for diagnosis in later phases of the disease. For the CDC single point serology test, the optimal timing for specimen collection is 2 to 8 weeks following cough onset, when the antibody titers are at their highest. However, you may perform serology on specimens collected up to 12 weeks following cough onset.
For more information, see the Pertussis Laboratory Information page or the Pertussis Laboratory Testing section of the Manual for the Surveillance of Vaccine-Preventable Diseases.
- Page last reviewed: August 7, 2017
- Page last updated: August 7, 2017
- Content source:




 ShareCompartir
ShareCompartir
