Surveillance and Reporting
On This Page
Trends
Pertussis is nationally-notifiable and clinicians should notify the appropriate health department of all patients with suspected pertussis. Similarly, diagnostic laboratories should notify health departments of all positive pertussis laboratory results. State health departments then report confirmed and probable pertussis cases to CDC through the National Notifiable Diseases Surveillance System (NNDSS). Although many pertussis cases are not diagnosed and therefore not reported, the surveillance system is useful for monitoring epidemiologic trends. The limitations of laboratory diagnostics make the clinical case definition essential to pertussis surveillance. It is important to determine duration of cough — specifically whether it lasts 14 days or longer — in order to determine if a person’s illness meets the definition of a clinical case.
To view the full list of information requested for pertussis case-patients, refer to the national pertussis surveillance worksheet [2 pages].
View larger image.
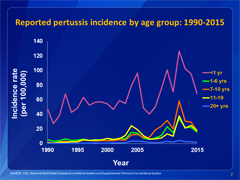
This graph shows reported pertussis incidence (per 100,000 persons) by age group in the United States from 1990–2015. Infants aged <1 year, who are at greatest risk for serious disease and death, continue to have the highest reported rate of pertussis.
View larger image.
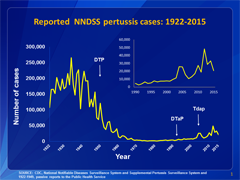
This graph illustrates the number of pertussis cases reported to CDC from 1922 to 2015. Following the introduction of pertussis vaccines in the 1940s when case counts frequently exceeded 100,000 cases per year, reports declined dramatically to fewer than 10,000 by 1965. During the 1980s pertussis reports began increasing gradually, and by 2015 more than 20,000 cases were reported nationwide. View data for this chart.
Case Definition
State health departments report pertussis cases to CDC NNDSS using a standardized case definition accepted by the Council of State and Territorial Epidemiologists. The most recent case definition (2014) for pertussis includes the following information:
2016 Provisional Surveillance Report
View provisional pertussis data for 2016, including case counts and incidence by state and age, DTaP vaccination history of cases, and pertussis-related deaths.
Clinical Case Definition
In the absence of a more likely diagnosis a cough illness lasting ≥2 weeks with one of the following symptoms:
- Paroxysms of coughing, OR
- Inspiratory “whoop,” OR
- Posttussive vomiting, OR
- Apnea (with or without cyanosis) (FOR INFANTS AGED < 1 YEAR ONLY)
Laboratory Criteria for Diagnosis
- Isolation of Bordetella pertussis from clinical specimen
- Positive polymerase chain reaction (PCR) for B. pertussis
Epidemiologic Linkage
Contact with a laboratory-confirmed case of pertussis*.
Case Classification
Probable:
- Meets the clinical case definition, is not laboratory confirmed, and is not epidemiologically linked to a laboratory-confirmed case, OR
- FOR INFANTS AGED < 1 YEAR ONLY:
- Acute cough illness of any duration with at least one of the following signs or symptoms:
- Paroxysms of coughing, OR
- Inspiratory “whoop”, OR
- Posttussive vomiting, OR
- Apnea (with or without cyanosis)
AND
- Polymerase chain reaction (PCR) positive for pertussis, OR
- Acute cough illness of any duration with at least one of the following signs or symptoms:
- FOR INFANTS AGED < 1 YEAR ONLY:
- Acute cough illness of any duration with at least one of the following signs or symptoms:
- Paroxysms of coughing, OR
- Inspiratory “whoop”, OR
- Posttussive vomiting, OR
- Apnea (with or without cyanosis)
AND
- Contact with a laboratory-confirmed case of pertussis
- Acute cough illness of any duration with at least one of the following signs or symptoms:
Confirmed:
- Acute cough illness of any duration with isolation of B. pertussis from a clinical specimen, OR
- Meets the clinical case definition AND is polymerase chain reaction (PCR) positive for pertussis, OR
- Meets the clinical case definition AND had contact with a laboratory-confirmed case of pertussis
Case Classification Comments:
*Note: An illness meeting the clinical case definition should be classified as “probable” rather than “confirmed” if it occurs in a patient who has contact with an infant aged < 1 year who is PCR positive for pertussis and has ≥ 1 sign or symptom and cough duration < 14 days (classified as “probable” case).
Enhanced Pertussis Surveillance (EPS)
CDC has partnered with seven states (CO, CT, GA, MN, NM, NY, and OR) participating in the Emerging Infections Program (EIP) network to conduct enhanced surveillance of pertussis and other Bordetella species. EPS sites conduct enhanced case ascertainment and augmented data collection. This additional information goes beyond what CDC receives through NNDSS nationally. Participating sites collect isolates and specimens, when available, for further characterization at the CDC Pertussis and Diphtheria Laboratory. EPS sites also provide the infrastructure for conducting pertussis special studies, including those aimed at evaluating pertussis prevention and control strategies.
Pertussis Surveillance Reports
- 2016 Provisional Pertussis Surveillance Report [1 page]
- 2015 Final Pertussis Surveillance Report [1 page]
- 2014 Final Pertussis Surveillance Report [1 page]
- 2013 Final Pertussis Surveillance Report [1 page]
- 2012 Final Pertussis Surveillance Report [1 page]
References
- National Notifiable Diseases Surveillance System (NNDSS)
2014 Case Definition: Pertussis (Bordetella pertussis) (Whooping Cough) - Council of State and Territorial Epidemiologists (CSTE) [10 pages]
Revision of the pertussis surveillance case definition to more accurately capture the burden of disease among infants < 1 year of age. Position statement 13-ID-15.
- Page last reviewed: August 7, 2017
- Page last updated: August 7, 2017
- Content source:




 ShareCompartir
ShareCompartir