Notes from the Field: Pediatric Emergency Department Visits for Buprenorphine/Naloxone Ingestion — United States, 2008–2015
Weekly / October 21, 2016 / 65(41);1148–1149
Daniel S. Budnitz, MD1; Maribeth C. Lovegrove, MPH1; Mathew R.P. Sapiano, PhD1; Justin Mathew, PharmD2; Scott R. Kegler, PhD3; Andrew I. Geller, MD1; Christian Hampp, PhD2 (View author affiliations)
View suggested citationExpanding access to office-based medication-assisted treatment with buprenorphine/naloxone for opioid dependence is a key part of the national strategy to address the opioid abuse epidemic (1). However, as buprenorphine/naloxone prescribing increased, emergency department (ED) visits and hospitalizations for unsupervised ingestions by young children began to increase, with buprenorphine/naloxone ingestions becoming the most common cause of hospitalization for medication ingestions by young children during 2010–2011 (2). Buprenorphine ingestions might be asymptomatic or can cause drowsiness, vomiting, or respiratory depression, which if untreated can result in death (3). Buprenorphine/naloxone was available only as tablets in multidose child-resistant bottles (Suboxone) until late 2010, when film strips packaged in unit-dose, child-resistant pouches were introduced. In 2013, tablets became available in unit-dose packaging (Zubsolv). Because unit-dose, child-resistant packaging encloses each dose until opened, it might limit unintended ingestions by young children compared with traditional child-resistant bottles that must be resecured after every use (4). This study compared ED visits for pediatric buprenorphine/naloxone ingestions before and after these product packaging/formulation changes.
Rates of ED visits for ingestions by children aged <6 years were calculated for the years 2008–2015 from estimates of ED visits for buprenorphine/naloxone ingestions (National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance [NEISS-CADES] project) and dispensed outpatient prescriptions (IMS Health: National Prescription Audit) (5). NEISS-CADES and IMS Health are national samples, with each case or prescription weighted to allow calculation of nationally representative estimates. A two-tailed test was used to evaluate any change in rates over the study period.
The estimated number of dispensed buprenorphine/naloxone prescriptions nearly tripled from 2008 (3,178,571) to 2015 (9,122,150). During 2008–2010, nearly all (97.6%) buprenorphine/naloxone prescriptions were dispensed as tablets in multidose bottles; by 2013–2015, most (86.9%) prescriptions were dispensed as unit-dose packaged tablets or film strips (Figure).
Based on 183 cases, there were an estimated 8,136 (95% confidence interval [CI] = 4,892–11,380) ED visits for buprenorphine/naloxone ingestions by children aged <6 years from 2008–2015. Three fourths of visits (75.4%; CI = 67.5%–83.2%) involved children aged 1 or 2 years, and half the visits (50.5%; CI = 36.6%–64.5%) involved boys. Most visits required hospitalization (61.6%; CI = 46.7%–76.5%). During 2008–2010, there were an estimated 1,246 ED visits (CI = 662–1,830) annually for buprenorphine/naloxone ingestions by children aged <6 years, compared with an estimated 799 visits (CI = 324–1,274) annually during 2013–2015. Accounting for prescribing frequency, ED visits for unsupervised buprenorphine/naloxone ingestions declined 65.3%, from an estimated 28.2 ED visits per 100,000 dispensed prescriptions during 2008–2010 to an estimated 9.8 per 100,000 dispensed prescriptions during 2013–2015 (p = 0.011).
The approximate two thirds reduction in the rate of ED visits by children for buprenorphine/naloxone ingestions as the proportion of prescriptions dispensed in unit-dose packaging increased to over 80%, suggests that packaging/formulation changes might reduce pediatric ingestions. A study of poison center calls for pediatric buprenorphine/naloxone exposures also found a significantly lower rate of calls involving film strips in unit-dose packaging, compared with tablets in multidose bottles (6). Other factors potentially contributing to the rate reduction include increased counseling of patients on safe use and storage (7) and a decline in pediatric medication ingestions overall (22% from 2010 to 2013) (8).
Although substantially decreased, ED visits for pediatric ingestions of buprenorphine/naloxone were not eliminated after widespread adoption of unit-dose, child-resistant packaging. One explanation might be that some patients using buprenorphine/naloxone for medication-assisted treatment divide doses rather than consuming the entire unit, leaving unused partial doses accessible to children. In addition, the proportion of buprenorphine/naloxone prescriptions dispensed in unit-dose packaging began to decline at the end of 2013, reflecting the introduction of generic buprenorphine/naloxone tablets packaged in multidose bottles. Citing concern for pediatric exposures, the Massachusetts Office of Medicaid made unit-dose packaged products available to those in households with children aged <6 years (9). At least one manufacturer of the generic product has voluntarily transitioned to unit-dose packaging, but others continue to use multidose bottles (7).
To improve access to medication-assisted treatment, the U.S. Department of Health and Human Services nearly tripled the maximum patient limit for buprenorphine prescribers in July 2016 (1). As prescribing increases, and if multidose bottles again become the predominant form of packaging, it will be important to monitor the rate of ED visits for pediatric buprenorphine ingestions and respond if the rate increases.
Acknowledgment
Grace Chai, Office of Surveillance and Epidemiology, Center for Drug Evaluation and Research, Food and Drug Administration.
Corresponding author: Daniel S. Budnitz, dbudnitz@cdc.gov, 404-498-0634.
1Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, CDC; 2Office of Surveillance and Epidemiology, Center for Drug Evaluation and Research, Food and Drug Administration; 3Division of Research, Analysis, and Practice Integration, National Center for Injury Prevention and Control, CDC.
References
- Medication assisted treatment for opioid use disorders. 42 C.F.R. 8. (2016). https://www.federalregister.gov/articles/2016/07/08/2016-16120/medication-assisted-treatment-for-opioid-use-disorders
- CDC. Notes from the Field: emergency department visits and hospitalizations for buprenorphine ingestion by children—United States, 2010–2011. MMWR Morb Mortal Wkly Rep 2013;62:56. PubMed
- Kim HK, Smiddy M, Hoffman RS, Nelson LS. Buprenorphine may not be as safe as you think: a pediatric fatality from unintentional exposure. Pediatrics 2012;130:e1700–3. CrossRef PubMed
- Budnitz DS, Salis S. Preventing medication overdoses in young children: an opportunity for harm elimination. Pediatrics 2011;127:e1597–9. CrossRef PubMed
- IMS Health. National prescription audit: January 2008 through December 2015. Collegeville, PA: IMS Health; 2016.
- Lavonas EJ, Banner W, Bradt P, et al. Root causes, clinical effects, and outcomes of unintentional exposures to buprenorphine by young children. J Pediatr 2013;163:1377–83.e1–3. CrossRef
- Buprenorphine-Containing Transmucosal Products for Opioid Dependence Companies. Risk evaluation and mitigation strategy. Medication guides. https://www.btodrems.com/SitePages/MedicationGuides.aspx
- Lovegrove MC, Weidle NJ, Budnitz DS. Trends in emergency department visits for unsupervised pediatric medication exposures, 2004–2013. Pediatrics 2015;136:e821–9. CrossRef PubMed
- The Commonwealth of Massachusetts Executive Office of Health and Human Services. Suboxone film and unintentional pediatric exposures (prescriber letter). Quincy, MA: The Commonwealth of Massachusetts Executive Office of Health and Human Services; 2012. http://www.mass.gov/eohhs/docs/masshealth/pharmacy/suboxone-film-ped-exposures.pdf
 FIGURE. Estimated rate of emergency department (ED) visits for unsupervised buprenorphine/naloxone ingestions by children aged <6 years per 100,000 dispensed prescriptions, compared with estimates of the percentage of outpatient buprenorphine/naloxone prescriptions dispensed in unit-dose packaging — United States, 2008–2015*
FIGURE. Estimated rate of emergency department (ED) visits for unsupervised buprenorphine/naloxone ingestions by children aged <6 years per 100,000 dispensed prescriptions, compared with estimates of the percentage of outpatient buprenorphine/naloxone prescriptions dispensed in unit-dose packaging — United States, 2008–2015*
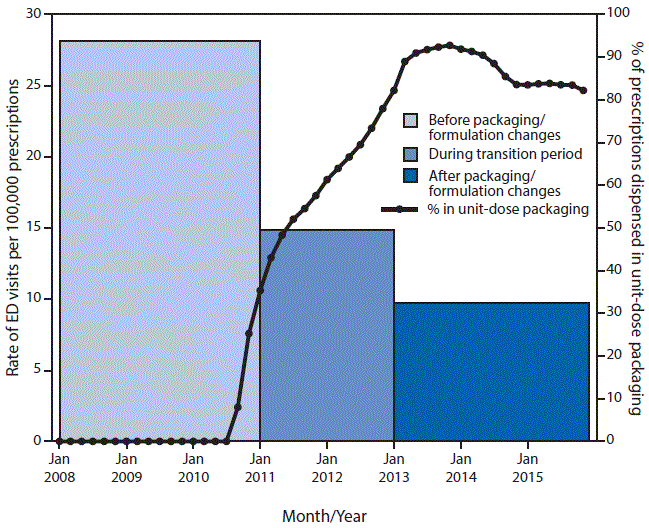
* Estimates of ED visits for pediatric buprenorphine/naloxone ingestions were based on 2008–2015 data from the National Electronic Injury Surveillance System-Cooperative Adverse Drug Event Surveillance project. Estimates of dispensed prescriptions and the percentage dispensed in unit-dose packaging were based on data from the IMS Health National Prescription Audit (2008–2015). Key dates of product changes were as follows: Suboxone buprenorphine/naloxone film in unit-dose packaging available (October 2010); first generic buprenorphine/naloxone products available as tablets in multidose bottles (February 2013); Suboxone buprenorphine/naloxone tablets in multidose bottles discontinued (March 2013); first buprenorphine/naloxone tablets (Zubsolv) available in unit-dose packaging (September 2013).
Suggested citation for this article: Budnitz DS, Lovegrove MC, Sapiano MR, et al. Notes from the Field: Pediatric Emergency Department Visits for Buprenorphine/Naloxone Ingestion — United States, 2008–2015. MMWR Morb Mortal Wkly Rep 2016;65:1148–1149. DOI: http://dx.doi.org/10.15585/mmwr.mm6541a5.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: August 17, 2017
- Page last updated: August 17, 2017
- Content source:


 ShareCompartir
ShareCompartir