Declines in Opioid Prescribing After a Private Insurer Policy Change — Massachusetts, 2011–2015
Weekly / October 21, 2016 / 65(41);1125–1131
Macarena C. García, DrPH1; Anton B. Dodek, MD2; Tom Kowalski2; John Fallon, MD2; Scott H. Lee, PhD1; Michael F. Iademarco, MD1; John Auerbach, MBA3; Michele K. Bohm, MPH4 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Overdose deaths involving opioid pain medications have reached epidemic levels in the United States, in part because of high opioid prescribing rates. Health systems and insurers play an important role in the delivery and management of pain care.
What is added by this report?
A private insurer implemented a comprehensive opioid utilization policy that included treatment plans, risk assessments, patient-provider agreements, requirements for dispensing from a single pharmacy, prior authorizations, quantity limits, and a ban on mail-order opioid prescriptions. Following implementation, monthly opioid prescribing rates and percentage of members with opioid prescriptions declined significantly.
What are the implications for public health practice?
Public health agencies and private health insurers can collaborate to ensure that both public and private insurers promote best practices in opioid prescribing.
Overdose deaths involving opioid pain medications are epidemic in the United States, in part because of high opioid prescribing rates and associated abuse of these drugs (1). In 2014, nearly 2 million U.S. residents either abused or were dependent on prescription opioids (2). In Massachusetts, unintentional opioid-related overdose deaths, including deaths involving heroin, increased 45% from 2012 to 2013.* In 2014, the rate of these deaths reached 20.0 per 100,000, nearly 2.5 times higher than the U.S. rate overall (3,4). On July 1, 2012, Blue Cross Blue Shield of Massachusetts (BCBSMA), the largest insurer in the state with approximately 2.8 million members,† implemented a comprehensive opioid utilization program after learning that many of its members were receiving new prescriptions with a >30-day supply of opioids. The 2016 CDC Guideline for Prescribing Opioids for Chronic Pain recommends avoiding opioids as a first-line therapy for chronic pain and limiting quantities when initiating opioids for acute pain (5). CDC analyzed BCBSMA prescription claims data for the period 2011–2015 to assess the effect of the new utilization program on opioid prescribing rates. During the first 3 years after policy implementation, the average monthly prescribing rate for opioids decreased almost 15%, from 34 per 1,000 members to 29. The percentage of BCBSMA members per month with current opioid prescriptions also declined. The temporal association between implementation of the program and statistically significant declines in both prescribing rates and proportion of members using opioids suggests that the BCBSMA initiative played a role in reducing the use of prescription opioids among its members. Public and private insurers in the United States could benefit from developing their own best practices for prescription opioid utilization that ensure accessible pain care, while reducing the risk for dependence and abuse associated with these drugs.
In 2012, BCBSMA analyzed its 2011 pharmacy claims data to determine the number of members receiving large quantities of opioid prescriptions from multiple providers. In 2011, approximately 30,000 members received new prescriptions of short-acting opioids with a >30-day supply; 25% of these members obtained opioid prescriptions from multiple providers. BCBSMA’s opioid utilization program was developed collaboratively among an extensive network of stakeholders, including physicians, nurses, pharmacists, actuaries, lawyers, data analysts, medical societies, medical and pharmacy boards, the Massachusetts Pain Initiative, and the top 10 opioid-dispensing pharmacies in Massachusetts (Box).
The BCBSMA prescription opioid utilization program was designed around expert-defined best practices for opioid prescribing that include formal agreements between patient and provider, a requirement for BCBSMA approval prior to dispensing new opioid prescriptions, and quantity limits.§ The program requires providers to conduct a risk assessment for abuse that the patient must sign. Physicians and patients work together to develop a treatment plan that considers options other than prescription opioids. When the decision to prescribe opioids is made, a formal agreement between patient and prescriber outlines specific behaviors expected of both parties. In addition, the prescriber must provide a diagnosis and rationale for prescribing an opiate as part of the prior authorization process. BCBSMA coverage requires prior authorization (including review by a BCBSMA clinician who then notifies the pharmacy) before dispensing new short-acting opioid prescriptions with a >30-day supply and for all new long-acting opioid prescriptions. Pharmacy mail orders are not permitted. If opioid misuse is suspected or if coordination of care among multiple providers is indicated, patients might be assigned a single pharmacy to dispense all opioid prescriptions. Identified patients with chronic pain are referred to case managers who advise on nonopioid therapies. Oncology patients and terminally ill persons are exempt from the requirements for prior authorization for new prescriptions. Members continue to have coverage for physical therapy, pain management, addiction treatment, chiropractic services, and cognitive behavioral therapy.
A retrospective analysis was conducted using BCBSMA prescription claims data from the period July 2011–June 2015. The pre-implementation period was defined as July 1, 2011–June 30, 2012, and the postimplementation period was defined as July 1, 2012–June 30, 2015. All data were deidentified before analysis. Average monthly prescribing rates per 1,000 members and percentage of members with opioid prescriptions were calculated for short-acting,¶ long-acting,** and for both opioid types combined. Average monthly counts of opioid prescriptions for oncology members were also tabulated. To assess the effects of the opioid utilization program, preprogram and postprogram rates were modeled using an interrupted time series analysis. Outcomes were the proportions of members with prescriptions per month and number of prescriptions per total members per month; changes in rates were estimated by two regression coefficients, one for each period. Differences were considered statistically significant if the 95% confidence interval for the coefficient corresponding to the difference in slopes excluded zero. Prescriptions for buprenorphine, indicated for the treatment of opioid use disorder, were examined separately to determine how prior authorization requirements impact dispensing of drugs used in medication-assisted treatment. Changes in prescribing are reported for all commercial members eligible for BCBSMA pharmacy benefits (excluding patients receiving Medicare benefits).
An average of 1.5 million commercial members with pharmacy benefits were enrolled in BCBSMA each month during the study period. The average monthly prescribing rate for all opioids decreased 14.7%, from 34 per 1,000 before implementation of the program to 29 after implementation (Table 1). Although the average monthly prescribing rates for long-acting opioids remained constant at three per 1,000 members before and after program implementation periods, the average monthly prescribing rate for short-acting opioids decreased 16.1%, from 31 to 26 per 1,000 members (Figure 1). Similarly, while the percentage of all members with a long-acting opioid prescription decreased 8.3%, from 0.24% per month to 0.22%, the percentage of members with short-acting opioid prescriptions decreased 12.9%, from 2.49% per month to 2.17% after program implementation (Figure 2). The number of members with cancer diagnoses was not available to calculate opioid prescribing rates; however, the average monthly number of opioid prescriptions dispensed to members with cancer diagnoses declined 9% following program implementation, which was less than among all members.
Results of the interrupted time series analysis showed a 6%–9% annual decline in the percentage of members on short-acting and long-acting opioid prescriptions and in opioid prescribing rates after implementation of the opioid utilization program compared with the pre-implementation period (Table 2). All differences were statistically significant, regardless of medication type. Overall, the estimated quantity of opioids dispensed before and after implementation of the program indicate that approximately 21 million fewer opioid doses were dispensed in the first 3 years after implementation. Data on prior authorization requirements for buprenorphine indicated for the treatment of opioid use disorder, show that 17% of members with these prescriptions never sought subsequent authorization to fill them following a pharmacy declining to fill a prescription.
Discussion
After implementation of an opioid utilization program in July 2012, the number of opioid prescriptions and the percentage of members with an opioid prescription significantly decreased among BCBSMA members. However, it is possible that other events, such as changes in policies and media coverage, contributed to the decline. Although the declines in average monthly prescribing rate and the percentage of BCBSMA members with opioid prescriptions appear modest, these data represent significant changes for a 2.8 million-member health plan. In the postimplementation period, the average monthly number of prescriptions for short-acting and long-acting opioids decreased by 14,000. The prior authorization requirement for new short-acting opioid prescriptions for >30 days prompts providers to evaluate the medical necessity of initiating opioids for extended periods, along with the concomitant risks. The decreases in dispensed opioids were highest for short-acting opioids, which also account for most of the opioid prescriptions. In accordance with a Massachusetts statute, the prior authorization requirement was changed to >21 days in August 2016.††
For nearly one in five patients, the pharmacy did not fill a buprenorphine prescription because it did not meet criteria and the patient did not subsequently seek authorization. The rejection might have occurred because the provider wrote the prescription for an off-label use or because the daily dosage was not within recommended parameters. Additional analyses by BCBSMA found that nearly one third of patients were receiving these prescriptions from multiple providers, indicating a potential problem with poorly coordinated care. Whereas prior authorization might prevent misuse or diversion of buprenorphine, it might also be a barrier to medication-assisted treatment. Insurers can weigh the benefits and harms of requiring prior authorization for drugs used as part of medication-assisted treatment to determine what is most effective for their member population.
Although oncology patients were exempt from prior authorization requirements for new prescription opioids, the number of opioid prescriptions also declined among these patients following program implementation. Insurers frequently observe a sentinel effect following a new drug utilization program in which provider behaviors extend to their entire patient populations (6). Effective management of pain is a core component of quality end-of-life care and care for patients with serious advanced illness. To avoid unintentionally limiting access to pain medication for these patients, insurers can evaluate how policies affect this population to ensure that comprehensive care addresses their specific needs, including pain management. Data are not available on the impact of the decrease in prescribing among BCBSMA oncology patients on their pain management and functioning. However, in the 4 years since program launch, the only appeal of a claim related to the insurer’s policy resulted from a clerical error, suggesting that these members continue to receive medically appropriate access to pain medication.
The findings in this report are subject to at least four limitations. First, rates for nononcology opioid prescriptions might be underestimated by the inclusion of oncology members in the denominator, but the impact on trends is likely minimal because of the relatively small number of members who are oncology patients. Second, the role of other factors that potentially affected prescribing rates during this period (e.g., media coverage of opioids, increased use of the prescription monitoring program, and overlapping policy changes) could not be evaluated. In 2012, for example, the Massachusetts state legislature provided a statutory directive to address prescription drug abuse (7). Effective October 2014, the Drug Enforcement Administration rescheduled hydrocodone combination products to schedule II,§§ which was followed by a decrease in hydrocodone prescribing at the national level (8). Although tramadol is a frequently prescribed opioid, it was excluded from the program. As a Schedule IV opioid, tramadol has a lower potential for abuse than Schedule II and Schedule III opioids; it is possible that providers, cognizant of the risks associated with opioids, altered their prescribing behaviors and substituted tramadol where possible. However, tramadol prescribing data from BCBSMA show neither an increasing nor decreasing trend, indicating tramadol substitution was not likely. Third, these results reflect a privately insured population and might not be generalizable to other populations, including persons covered under public health plans. Finally, it is not known from these data how patient pain and function were affected by limiting access to opioid prescriptions.
State and federal initiatives to address the opioid epidemic in the United States have been implemented in the past several years, with some resulting in reduced opioid prescribing (8,9). The U.S. Department of Health and Human Services initiative targets three priority areas: improving opioid prescribing practices, distribution of naloxone to reverse overdoses, and access to medication-assisted treatment (10). The significant decrease in dispensing of opioids immediately after the implementation of the BCBSMA opioid utilization program suggests that this intervention played a role in the reduction of the observed monthly prescription rate. As part of quality improvement efforts, public and private insurers can implement policies that promote best practices in opioid prescribing to reduce risk among their members while ensuring access to appropriate pain management. The CDC Guideline for Prescribing Opioids for Chronic Pain (5), released March 2016, supports this effort and provides a comprehensive list of recommendations that can inform insurer opioid utilization programs and policies.
Acknowledgments
Mary Beth Erwin, MPH, Peter Lakin, MBA, David Lynch, Blue Cross Blue Shield of Massachusetts; Julie Kitson, PharmD, Express Scripts.
Corresponding author: Macarena C. García, mcgarcia@cdc.gov, 404-539-4410.
1Center for Surveillance, Epidemiology, and Laboratory Services, CDC; 2Blue Cross Blue Shield of Massachusetts; 3Office of the Associate Director for Policy, Office of the Director, CDC; 4Division of Unintentional Injury Prevention, National Center for Injury Prevention and Control, CDC.
† Total state population = 6.79 million (2015 census).
§https://www.nhpf.org/uploads/Handouts/Kowalski-slides_06-20-14.pdf.
¶ Short-acting opioids in the BCBSMA opioid utilization program include short-acting formulations of acetaminophen/caffeine/dihydrocodeine, acetaminophen/codeine, acetaminophen/hydrocodone, acetaminophen/oxycodone, alfentanil, aspirin/caffeine/dihydrocodeine, aspirin/hydrocodone, aspirin/oxycodone, codeine, fentanyl, hydromorphone, ibuprofen/hydrocodone, ibuprofen/oxycodone, levorphanol tartrate, meperidine, morphine, oxycodone, oxymorphone, and tapentadol.
** Long-acting opioids in the BCBSMA opioid utilization program include extended-release formulations and naturally long-acting opioids: acetaminophen/oxycodone, buprenorphine (transdermal), fentanyl, hydrocodone, hydromorphone, methadone, morphine, oxycodone, oxymorphone, tapentadol.
††https://malegislature.gov/Bills/189/House/H4056.
§§ Imposing the same restrictions that apply to pure hydrocodone, as well as oxycodone and morphine.
References
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2016;64:1378–82. CrossRef PubMed
- Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug Use and Health: summary of national findings. NSDUH Series H-46. Rockville, MD: US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; 2013.
- National Center for Health Statistics. Multiple cause of death, 1999–2014. US Department of Health and Human Services, CDC, National Center for Health Statistics. https://wonder.cdc.gov/mcd-icd10.html
- Massachusetts Department of Public Health. Data brief: opioid-related overdose deaths among Massachusetts residents. August 2016. Boston, MA: Massachusetts Department of Public Health; 2016. http://www.mass.gov/eohhs/docs/dph/quality/drugcontrol/county-level-pmp/opioid-related-overdose-deaths-among-ma-residents-august-2016.pdf
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016;65(No. RR-1). CrossRef PubMed
- LaPensee KT. Analysis of a prescription drug prior authorization program in a Medicaid health maintenance organization. J Manag Care Pharm 2003;9:36–44. CrossRef PubMed
- Massachusetts Department of Public Health. Response to the Massachusetts opioid prescription drug epidemic. 2014 report of best practices. Boston, MA: Massachusetts Department of Public Health; 2015. http://www.mass.gov/eohhs/docs/dph/quality/drugcontrol/best-practices/best-practices-workgroup-report.pdf
- Jones CM, Lurie PG, Throckmorton DC. Effect of US Drug Enforcement Administration’s rescheduling of hydrocodone combination analgesic products on opioid analgesic prescribing. JAMA Intern Med 2016;176:399–402. CrossRef PubMed
- Johnson H, Paulozzi L, Porucznik C, Mack K, Herter B; Hal Johnson Consulting; Division of Disease Control and Health Promotion, Florida Department of Health. Decline in drug overdose deaths after state policy changes – Florida, 2010-2012. MMWR Morb Mortal Wkly Rep 2014;63:569–74. PubMed
- US Department of Health and Human Services. ASPE issue brief. Opioid abuse in the U.S. and HHS actions to address opioid-drug related overdoses and deaths. March 26, 2015. Washington, DC: US Department of Health and Human Services; 2015. https://aspe.hhs.gov/basic-report/opioid-abuse-us-and-hhs-actions-address-opioid-drug-related-overdoses-and-deaths
 BOX. Development plan for the Blue Cross Blue Shield of Massachusetts (BCBSMA) opioid utilization program
BOX. Development plan for the Blue Cross Blue Shield of Massachusetts (BCBSMA) opioid utilization program
-
Designed around expert-defined best practices for opioid prescribing
-
Collaborative effort involving an extensive network of stakeholders including:
-
Physicians, nurses, and pharmacists
-
Actuaries
-
Lawyers
-
Data analysts
-
Medical societies
-
Medical and pharmacy boards
-
Massachusetts Pain Initiative, a patient advocacy group
-
Top 10 opioid-dispensing pharmacies in Massachusetts
-
Program elements
-
Patient and provider work together to manage pain safely and effectively
-
Providers conduct a risk assessment for abuse that the patient must sign.
-
Physicians and patients develop a treatment plan that considers options other than prescription opioids.
-
If opioids are prescribed, a formal agreement between patient and prescriber outlines behavior expected of both parties.
-
When opioid misuse is suspected or when coordination of care among multiple providers is indicated, patients might be assigned a single pharmacy to dispense all opioid prescriptions.
-
-
Parameters established by BCBSMA
-
Prior authorization required for new short-acting opioid prescriptions with more than a 30-day supply.*
-
Prior authorization required for all new long-acting opioid prescriptions.*
-
Pharmacy mail orders are no longer permitted.
-
All patients with chronic pain are referred to case management who advise them on nonopioid therapies.
-
All members have coverage for physical therapy, pain management, addiction treatment, chiropractic services, and cognitive behavioral therapy.
-
* Members who are oncology patients or terminally ill are exempt from the requirements of prior authorization for new prescriptions.
 TABLE 1. Average monthly opioid prescribing rates and percentage of BCBSMA members with opioid prescriptions per month before and after implementation of an opioid utilization program — Massachusetts, July 2011–June 2012 (pre-implementation) and July 2012–June 2015 (postimplementation)
TABLE 1. Average monthly opioid prescribing rates and percentage of BCBSMA members with opioid prescriptions per month before and after implementation of an opioid utilization program — Massachusetts, July 2011–June 2012 (pre-implementation) and July 2012–June 2015 (postimplementation)
| Opioid prescription drugs | Average monthly prescribing rate (no. of prescriptions per 1,000 members per month) |
Average percentage of members with prescriptions per month | ||
|---|---|---|---|---|
| All opioids | Pre-implementation | Postimplementation | Pre-implementation | Postimplementation |
| 34 | 29 | 2.58 | 2.24 | |
| Long-acting | 3 | 3 | 0.24 | 0.22 |
| Short-acting | 31 | 26 | 2.49 | 2.17 |
Abbreviation: BCBSMA = Blue Cross Blue Shield of Massachusetts.
 TABLE 2. Annual percentage change (APC) in member opioid prescription drug use before and after implementation of an opioid utilization program, by indicator — Blue Cross Blue Shield of Massachusetts, July 2011–June 2012 (pre-implementation) and July 2012–June 2015 (postimplementation)
TABLE 2. Annual percentage change (APC) in member opioid prescription drug use before and after implementation of an opioid utilization program, by indicator — Blue Cross Blue Shield of Massachusetts, July 2011–June 2012 (pre-implementation) and July 2012–June 2015 (postimplementation)
| Indicator | APC Pre-implementation | APC Postimplementation | APC difference (95% CI) |
|---|---|---|---|
| Members with SA opioid prescription | -0.898 | -7.217 | -6.319 (-7.354 to -5.284) |
| SA opioid prescription rate* | -2.248 | -8.312 | -6.064 (-6.993 to -5.134) |
| Members with LA opioid prescription (%) | 2.921 | -5.854 | -8.775 (-12.079 to -5.471) |
| LA opioid prescription rate* | 3.062 | -6.044 | -9.105 (-12.095 to -6.115) |
| Members with SA or LA opioid prescription (%) | -0.716 | -7.238 | -6.522 (-7.540 to -5.504) |
| SA or LA opioid prescription rate* | -1.781 | -8.104 | -6.322 (-7.210 to -5.435) |
Abbreviations: LA = long-acting; SA = short-acting.
* Number of prescriptions per 1,000 members.
 FIGURE 1. Average monthly prescribing rates* for opioids — Blue Cross Blue Shield of Massachusetts (BCBSMA), July 2011–June 2015†
FIGURE 1. Average monthly prescribing rates* for opioids — Blue Cross Blue Shield of Massachusetts (BCBSMA), July 2011–June 2015†
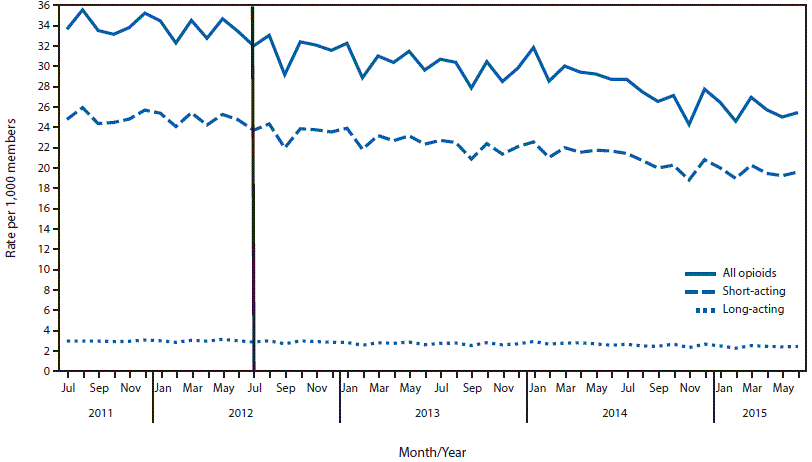
* Per 1,000 members. Based on BCBSMA total monthly member enrollment data.
† July 1, 2012, marked the start date of the BCBSMA opioid utilization program.
 FIGURE 2. Percentage of members with opioid prescriptions — Blue Cross Blue Shield of Massachusetts (BCBSMA), July 2011–June 2015*
FIGURE 2. Percentage of members with opioid prescriptions — Blue Cross Blue Shield of Massachusetts (BCBSMA), July 2011–June 2015*
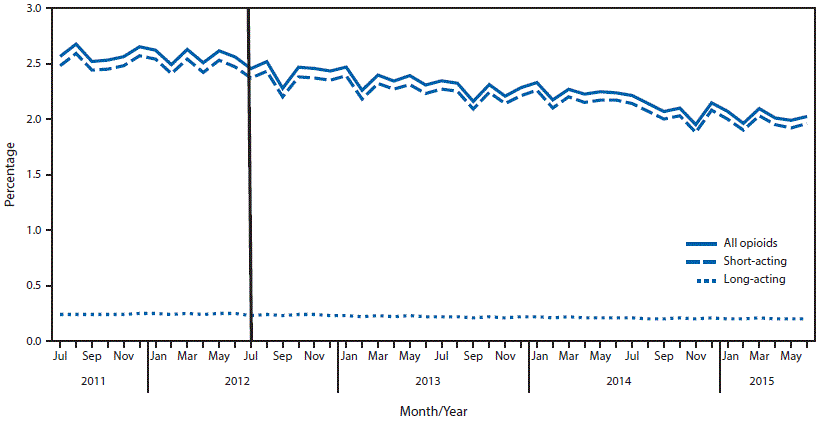
* July 1, 2012, marked the start date of the BCBSMA opioid utilization program.
Suggested citation for this article: García MC, Dodek AB, Kowalski T, et al. Declines in Opioid Prescribing After a Private Insurer Policy Change — Massachusetts, 2011–2015. MMWR Morb Mortal Wkly Rep 2016;65:1125–1131. DOI: http://dx.doi.org/10.15585/mmwr.mm6541a1.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: August 17, 2017
- Page last updated: August 17, 2017
- Content source:


 ShareCompartir
ShareCompartir