Cessation of Trivalent Oral Poliovirus Vaccine and Introduction of Inactivated Poliovirus Vaccine — Worldwide, 2016
Weekly / September 9, 2016 / 65(35);934–938
Lee M. Hampton, MD1; Margaret Farrell, MPH2; Alejandro Ramirez-Gonzalez, MPH3; Lisa Menning, MSc3; Stephanie Shendale, JD3; Ian Lewis4; Jennifer Rubin, MPH4; Julie Garon, MPH5; Jennifer Harris, PhD1; Terri Hyde, MD1; Steven Wassilak, MD1; Manish Patel, MD6; Robin Nandy, MD2; Diana Chang-Blanc, MPP3; Immunization Systems Management Group of the Global Polio Eradication Initiative (View author affiliations)
View suggested citationSummary
What is already known about this topic?
To address the risks posed by type 2 circulating vaccine-derived polioviruses, which have caused hundreds of paralytic poliomyelitis cases since 2006, the type 2 component of oral poliovirus vaccine was scheduled for global withdrawal through a synchronized switch from trivalent oral poliovirus vaccine (tOPV) to bivalent oral poliovirus vaccine (bOPV). All countries not already using inactivated polio vaccine (IPV) have committed to introducing it as part of their efforts to reduce risks associated with potential type 2 polio outbreaks after the switch.
What is added by this report?
All 155 countries and territories using OPV in their immunization programs in 2015 reported that they had completely ceased use of tOPV by mid-May 2016. As of August 31, 173 (89%) of 194 WHO countries had introduced inactivated polio vaccine (IPV) into their immunization programs despite a global shortage of IPV, although 29 of those countries are expected to deplete their supplies of IPV before being resupplied in 2017.
What are the implications for public health practice?
The cooperation of all OPV-using countries ending use of tOPV in a synchronized fashion is unprecedented. However, although the cessation of tOPV use is a major milestone toward the completion of the global effort to eradicate polio, vigilant surveillance for all polioviruses, including type 2 polioviruses, is still needed. Any tOPV found in a vaccine storage refrigerator or freezer in the future should be destroyed consistent with the Global Action Plan to Minimize Poliovirus Facility-Associated Risk. Any type 2 polioviruses detected in the future will require thorough investigation and an aggressive response.
Since the 1988 World Health Assembly resolution to eradicate poliomyelitis, transmission of the three types of wild poliovirus (WPV) has been sharply reduced (1). WPV type 2 (WPV2) has not been detected since 1999 and was declared eradicated in September 2015. Because WPV type 3 has not been detected since November 2012, WPV type 1 (WPV1) is likely the only WPV that remains in circulation (1). This marked progress has been achieved through widespread use of oral poliovirus vaccines (OPVs), most commonly trivalent OPV (tOPV), which contains types 1, 2, and 3 live, attenuated polioviruses and has been a mainstay of efforts to prevent polio since the early 1960s. However, attenuated polioviruses in OPV can undergo genetic changes during replication, and in communities with low vaccination coverage, can result in vaccine-derived polioviruses (VDPVs) that can cause paralytic polio indistinguishable from the disease caused by WPVs (2). Among the 721 polio cases caused by circulating VDPVs (cVDPVs*) detected during January 2006–May 2016, type 2 cVDPVs (cVDPV2s) accounted for >94% (2). Eliminating the risk for polio caused by VDPVs will require stopping all OPV use. The first stage of OPV withdrawal involved a global, synchronized replacement of tOPV with bivalent OPV (bOPV) containing only types 1 and 3 attenuated polioviruses, planned for April 18–May 1, 2016, thereby withdrawing OPV type 2 from all immunization activities (3). Complementing the switch from tOPV to bOPV, introduction of at least 1 dose of injectable, trivalent inactivated poliovirus vaccine (IPV) into childhood immunization schedules reduces risks from and facilitates responses to cVDPV2 outbreaks. All 155 countries and territories that were still using OPV in immunization schedules in 2015 have reported that they had ceased use of tOPV by mid-May 2016.† As of August 31, 2016, 173 (89%) of 194 World Health Organization (WHO) countries included IPV in their immunization schedules.§ The cessation of tOPV use is a major milestone toward the global goal of eradicating polio; however, careful surveillance for polioviruses and prompt, aggressive responses to polio outbreaks are still needed to realize a polio-free world.
Global Cessation of Use of Trivalent Oral Poliovirus Vaccine
Although the global cessation of tOPV use is essential for eliminating cVDPV2s, cessation of tOPV use carries some risks for facilitating the spread of undetected or newly emergent cVDPV2s among persons without immunity to type 2 poliovirus infections after the switch to bOPV (3–5). To stop the spread of existing cVDPV2s before the switch and to reduce risks for post-switch outbreaks (4), population immunity to type 2 poliovirus at the time of the switch was boosted through implementation of 116 supplemental immunization activities (SIAs¶) with tOPV in 42 OPV-using countries during November 2015–April 2016. Afghanistan, Nigeria, and Pakistan also conducted SIAs with IPV in selected regions before stopping tOPV use. In addition, the synchronized timing of the switch aimed to prevent exportations of type 2 polioviruses from areas continuing to use tOPV to neighboring areas that have ceased tOPV use (3,4). All 155 countries and territories that used OPV in 2015 reported that they had terminated use of tOPV by May 12, 2016 (Figure 1). To facilitate global cessation of tOPV use, all manufacturers of OPV ended production of tOPV before the switch, after several years of communications and close coordination with the Global Polio Eradication Initiative (GPEI).**
To reduce the risk for inadvertent or intentional use of tOPV after the switch, which could lead to the emergence of new cVDPV2s (5), a combination of external and in-country monitors visited >160,000 vaccine stores and service delivery points in countries and territories participating in the switch. Monitors verified the absence of tOPV from each area’s vaccine supply cold chain and helped ensure that any tOPV they found in the cold chain was removed. The monitors’ findings in each country and territory were reviewed by a validation committee, whose assessment of whether or not tOPV had been removed from the cold chain was provided to the national government and later transmitted to WHO. By August 31, 2016, all but two countries and territories that used OPV in 2015 had submitted validation reports to WHO.
Type 2 poliovirus strains held in research or manufacturing facilities also might cause polio outbreaks if released into a population. To prevent such outbreaks, countries should ensure that all remaining type 2 polioviruses, including WPV2s, VDPV2s, and the type 2 Sabin polioviruses used in tOPV and monovalent OPV type 2 (mOPV2), are destroyed or appropriately contained in certified poliovirus-essential facilities in accordance with the third Global Action Plan to Minimize Poliovirus Facility-Associated Risk (GAPIII) (3,6).
If type 2 poliovirus outbreaks do occur, GPEI has developed a response protocol and assembled a global stockpile of mOPV2, managed by the United Nations Children’s Fund and stored under containment conditions, to be released at the direction of the WHO Director-General. As of August 31, this stockpile contained approximately 36 million mOPV2 doses in finished vials. An additional 50 million mOPV2 doses will become available between September and December 2016, and another 50 million doses by March 2017. Hundreds of millions of doses stored in bulk form are also available for conversion into finished mOPV2 doses. GPEI has also created an IPV stockpile for use in outbreak responses. Surveillance for acute flaccid paralysis cases is supplemented by environmental surveillance for polioviruses in sewage in at least 36 countries to help identify and respond to the asymptomatic spread of type 2 polioviruses in those countries (7).
Global Introduction of Inactivated Poliovirus Vaccine
To further reduce the risk for type 2 poliovirus outbreaks after the cessation of tOPV use, the WHO Strategic Advisory Group of Experts (SAGE) on Immunization recommended in 2012 that all countries’ immunization schedules include at least 1 IPV dose (3). IPV protects against paralytic polio from type 2 polioviruses, might facilitate interruption of transmission during cVDPV2 outbreaks by enhancing immunologic response to mOPV2 and reducing the duration and amount of viral shedding, and aids in eradicating WPV by boosting immunity to types 1 and 3 polioviruses in persons who have received bOPV or tOPV (3).
Efforts to introduce IPV in the 126 countries using only OPV at the beginning of 2013 have been hampered by challenges manufacturers have experienced in scaling up production to meet the increased demand for IPV, as well as increased need for IPV in SIAs targeting WPV1 in countries where polio is endemic and the need to stockpile IPV for outbreak response. As of August 31, 2016, among the 126 countries using only OPV at the beginning of 2013, a total of 105 (83%) had introduced IPV, resulting in 173 (89%) of 194 WHO member states using IPV. However, 20 countries have had to delay introduction of IPV until adequate supplies of IPV become available, which is not likely before the fourth quarter of 2017 (Figure 2). In addition, 29 countries that previously introduced IPV are expected to run out of IPV nationwide before they receive their next supply of IPV in late 2017, and Cabo Verde has opted to postpone its introduction until 2017 to avoid a similar stock-out.††
In response to the IPV shortage, GPEI has set priorities for allocating the limited IPV supply. The highest priority countries for receipt of IPV are Afghanistan, Nigeria, and Pakistan because of ongoing indigenous WPV transmission. The second priority is the other 33 countries considered to be at high risk for cVDPV2 outbreaks. The third priority for IPV allocation is SIAs conducted in response to polio outbreaks, and the final priority is countries considered to be at low risk for polio outbreaks.§§ All countries considered to be at high risk for cVDPV2 outbreaks are providing IPV to infants through routine immunization service delivery. To use limited supplies of IPV efficiently, SAGE has recommended that countries consider administering 2 intradermal fractional doses of IPV to children eligible for IPV, instead of 1 full intramuscular dose (8). Two fractional doses of IPV, administered at separate visits, elicit a better immune response than a single full intramuscular dose of IPV, yet each fractional dose requires only one-fifth the volume of vaccine of a full intramuscular dose. Sri Lanka and India have begun administering 2 fractional doses of IPV to children through their routine immunization services.
Discussion
The synchronized global switch from tOPV to bOPV has gone smoothly based on the reported cessation of tOPV use in all countries and territories by mid-May 2016. The 721 cases of polio caused by cVDPV2s during 2006–2016 highlight both why the switch was necessary and why multiple precautions were taken to prevent cVDPV2s from emerging or spreading after the switch (2). Maintaining strong surveillance and response systems that can detect polioviruses, and responding promptly and aggressively when poliovirus is detected, will be essential for preserving and building upon the gains made against polio since 1988. The prompt detection and destruction of any tOPV vials found in the cold chain in the future, as well as of any mOPV2 vials found outside of the global mOPV2 stockpile after completion of an mOPV2 SIA, also will help to prevent new cVDPV2s from emerging in the future. Ultimately, the success of the withdrawal of tOPV and associated activities such as the tOPV and IPV SIAs held in the months before the switch and the global introduction of IPV will be measured by the number of polio cases caused by cVDPV2s that occur after tOPV withdrawal, with fewer cases indicating a greater success.
As of August 31, 2016, no new cVDPV outbreaks had been identified in 2016 (2). In April 2016, a cVDPV2 was identified in an environmental sample collected in March 2016 in northeastern Nigeria, before cessation of tOPV use, but genetic testing indicated that it is part of a known cVDPV2 lineage that was undetected after isolation from an environmental sample in early 2014 (9). Following the protocol for responding to detection of WPV2 after the switch and using the prepared mOPV2 stockpile, SIAs with mOPV2 were implemented in northeastern Nigeria after detection of the cVDPV2.¶¶ An SIA with fractional dose IPV is planned for the same area later in September, and SIAs with mOPV2 are planned for the high-risk neighboring countries of Cameroon, Chad, and Niger in October and November.*** The response to the identification in August of polio cases caused by WPV1 in northeastern Nigeria should lead to further strengthening of surveillance and, through vaccination, population immunity to polio infections in that area (10).
The introduction of IPV into the immunization schedules of 105 countries since 2013 is an important achievement, particularly given the challenges imposed by the global supply shortage. Continued external support for IPV introduction in countries that have not yet been able to introduce IPV but plan to do so once the supply shortages have been resolved and strengthening of routine immunization systems that distribute and administer IPV will help to maximize the benefit of IPV for all children.
The experience developed from tOPV cessation will contribute to the success of future efforts directed at the cessation of all OPV use, primarily the withdrawal of bOPV. The cooperation of all OPV-using countries and territories in ending tOPV use in a synchronized manner is an unprecedented public health achievement. This synchronized withdrawal of tOPV followed over 2 years of preparation by and communications among GPEI, its partner organizations, OPV manufacturers, and country and territorial governments, and was achieved by essential work performed by immunization workers in the countries and territories that stopped use of tOPV. Active support from senior leaders of GPEI and national ministries of health was critical, as was the cooperation of all OPV manufacturers in ceasing production and distribution of tOPV and ensuring the availability and timely delivery of bOPV. Combined with the eradication of WPV, the ultimate withdrawal of all OPV from use will enable the creation of a polio-free world.
Acknowledgments
Becky Maholland, Division of Emergency Operations Situational Awareness Geographic Information Systems, CDC.
Corresponding author: Lee M. Hampton, lhampton@cdc.gov, 404-639-4722.
1Global Immunization Division, CDC; 2Programme Division, United Nations Children’s Fund, New York; 3Immunization, Vaccines, and Biologicals Department, World Health Organization; 4Supply Division, United Nations Children’s Fund, New York; 5Emory University School of Medicine, Atlanta, Georgia; 6Task Force for Global Health, Decatur, Georgia.
References
- Morales M, Tangermann RH, Wassilak SGF. Progress toward polio eradication—worldwide, 2015–2016. MMWR Morb Mortal Wkly Rep 2016;65:470–3. CrossRef PubMed
- Jorba J, Diop OM, Iber J, Sutter RW, Wassilak SG, Burns CC. Update on vaccine-derived polioviruses—worldwide, January 2015–May 2016. MMWR Morb Mortal Wkly Rep 2016;65:763–9. CrossRef PubMed
- Global Polio Eradication Initiative. Polio eradication and endgame strategic plan 2013–2018. Geneva, Switzerland: World Health Organization; 2013. http://www.polioeradication.org/resourcelibrary/strategyandwork.aspx
- Duintjer Tebbens RJ, Hampton LM, Thompson KM. Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: risks of potential non-synchronous cessation. BMC Infect Dis 2016;16:231. CrossRef PubMed
- Duintjer Tebbens RJ, Hampton LM, Thompson KM. Implementation of coordinated global serotype 2 oral poliovirus vaccine cessation: risks of inadvertent trivalent oral poliovirus vaccine use. BMC Infect Dis 2016;16:237. CrossRef PubMed
- Previsani N, Tangermann RH, Tallis G, Jafari HS. World Health Organization guidelines for containment of poliovirus following type-specific polio eradication—worldwide, 2015. MMWR Morb Mortal Wkly Rep 2015;64:913–7. CrossRef PubMed
- Snider CJ, Diop OM, Burns CC, Tangermann RH, Wassilak SG. Surveillance systems to track progress toward polio eradication—worldwide, 2014–2015. MMWR Morb Mortal Wkly Rep 2016;65:346–51. CrossRef PubMed
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, April 2016—conclusions and recommendations. Wkly Epidemiol Rec 2016;91:265–84. PubMed
- Etsano A, Damisa E, Shuaib F, et al. Environmental isolation of circulating vaccine derived poliovirus after interruption of wild poliovirus transmission—Nigeria, 2016. MMWR Morb Mortal Wkly Rep 2016;65:770–3. CrossRef PubMed
- World Health Organization. Media centre: government of Nigeria reports 2 wild polio cases, first since July 2014. Geneva, Switzerland: World Health Organization; 2016. http://www.who.int/mediacentre/news/releases/2016/nigeria-polio/en/
* VDPVs are classified as cVDPVs if genetically linked examples of a VDPV strain are isolated from at least two persons who are not household contacts, from one person and at least one environmental surveillance (sewage) sample, or from two or more environmental surveillance samples collected from different environmental surveillance sites or collected from the same site more than 2 months apart.
† Five countries reported ceasing all regular use of any OPV between early 2015 and March 2016. The other 150 countries and territories still using OPV in April 2016 all reported ceasing use of tOPV by May 12, 2016.
§ WHO tracks progress in the introduction of new vaccines by the number of WHO member states that have introduced a given vaccine. However, because of the need for even higher precision in tracking cessation of tOPV use, seven countries and territories using OPV in 2015 that are not full WHO member states were included in efforts to track tOPV use in addition to 148 countries using OPV in 2015 that are full WHO member states.
¶ Supplemental immunization activities are mass vaccination campaigns conducted over a short period (days to weeks), in this case, in which a dose of OPV is administered to all children aged <5 years, regardless of previous vaccination history. Campaigns can be conducted nationally or subnationally, in portions of a country.
** The Global Polio Eradication Initiative coordinates the global effort to eradicate polio; WHO, the United Nations Children’s Fund, CDC, Rotary International, and the Bill and Melinda Gates Foundation are core partners.
†† Eight of these countries are Pacific island countries.
§§ Countries are considered to be at high risk for a cVDPV2 outbreak if they have had a cVDPV outbreak since 2000, have endemic WPV transmission, or have estimated routine immunization coverage of <80% for the third dose of a vaccine containing diphtheria, tetanus, and pertussis antigens.
¶¶ New type 2 ambiguous vaccine-derived polioviruses (aVDPV2s), which are VDPV2s that cannot be classified as either circulating VDPV2s or immunodeficiency-related VDPV2s (iVDPV2s) after adequate investigation, had been identified in 2016 in the Democratic Republic of Congo, Egypt, India, Kenya, Nigeria, Pakistan, Senegal, and Syria, as of August 31. However, these aVDPV2s have generally had relatively few genetic changes compared with the attenuated Sabin polioviruses in OPV they are descended from, indicating they were detected relatively soon after they emerged. All of the affected countries conducted SIAs with tOPV in 2016 in preparation for the switch. Specifically in response to the detections of these aVDPV2s, a localized SIA with tOPV was held in early May in Egypt, a localized fIPV SIA was held in June in India, and a series of mOPV2 SIAs were conducted in Nigeria. In addition, a localized mOPV2 SIA and a localized fIPV SIA are planned in Pakistan.
*** As of August 31, 2016, additional VDPV2s had been identified in Ukraine and Yemen and had not yet been classified as cVDPV2s, aVDPV2s, or iVDPV2s because they were under investigation.
 FIGURE 1. Countries and territories using oral poliovirus vaccine (OPV) — worldwide, 2015
FIGURE 1. Countries and territories using oral poliovirus vaccine (OPV) — worldwide, 2015
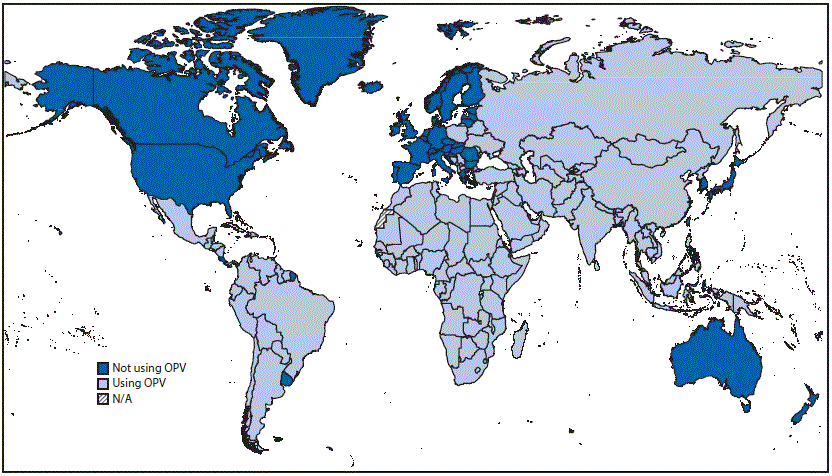
Abbreviation: N/A = not available.
Source: World Health Organization Immunization Repository.
 FIGURE 2. Status of introduction of inactivated poliovirus vaccine (IPV), by country — worldwide, August 31, 2016
FIGURE 2. Status of introduction of inactivated poliovirus vaccine (IPV), by country — worldwide, August 31, 2016
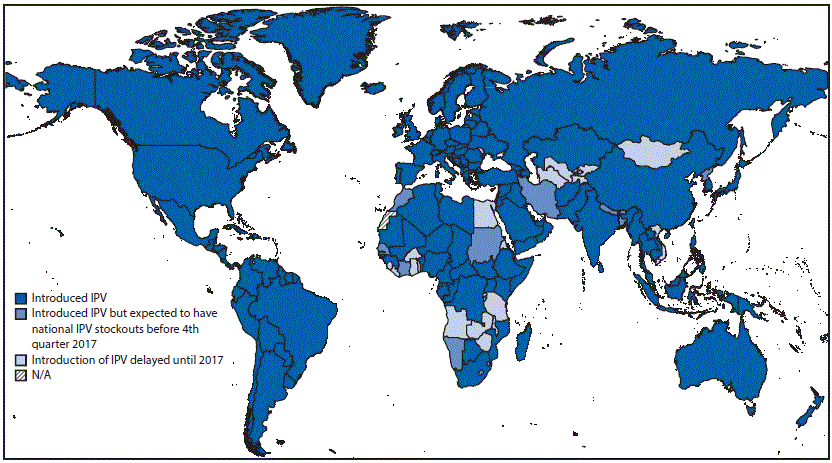
Abbreviation: N/A = not available.
Source: World Health Organization Immunization Repository.
Suggested citation for this article: Hampton LM, Farrell M, Ramirez-Gonzalez A, et al. Cessation of Trivalent Oral Poliovirus Vaccine and Introduction of Inactivated Poliovirus Vaccine — Worldwide, 2016. MMWR Morb Mortal Wkly Rep 2016;65:934–938. DOI: http://dx.doi.org/10.15585/mmwr.mm6535a3.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: August 17, 2017
- Page last updated: August 17, 2017
- Content source:


 ShareCompartir
ShareCompartir