Carbapenem-Resistant Enterobacteriaceae Transmission in Health Care Facilities — Wisconsin, February–May 2015
Weekly / September 2, 2016 / 65(34);906–909
Lina I. Elbadawi, MD1,2; Gwen Borlaug, MPH2; Kristin M. Gundlach3; Timothy Monson, MS3; David Warshauer, PhD3; Maroya S Walters, PhD4; Alexander Kallen, MD4; Christopher A. Gulvik, PhD4; Jeffrey P. Davis, MD2 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Carbapenem-resistant Enterobacteriaceae (CRE) are multidrug-resistant gram-negative bacilli that can cause infections associated with high case fatality rates, and are emerging as epidemiologically important health care–associated pathogens in the United States. Prevention of CRE transmission in health care settings is dependent on recognition of cases, isolation of colonized and infected patients, effective use of infection control measures, and the correct use of antibiotics.
What is added by this report?
Through the Wisconsin State Laboratory of Hygiene laboratory-based CRE surveillance program, which requests all clinical microbiology laboratories to submit carbapenem-nonsusceptible Enterobacteriaceae isolates for molecular testing by one or more methods (e.g., polymerase chain reaction [PCR], pulsed-field gel electrophoresis [PFGE], and whole genome sequencing [WGS]), a cluster of CRE infections among four hospital inpatients at two southeastern Wisconsin hospitals was discovered. At the time, personnel at the two implicated hospitals were not previously aware of the possibility of transmission of CRE among their patients.
What are the implications for public health practice?
The use of molecular technologies, including PCR testing, PFGE, and WGS, can lead to detection of transmission events and interruption of transmission by uncommon and multidrug-resistant organisms. Public health and other programs that include antibiotic stewardship and antimicrobial resistance monitoring might benefit from data generated by molecular testing of multidrug-resistant organisms to enhance detection of intra- and interfacility transmission events.
Carbapenem-resistant Enterobacteriaceae (CRE) are multidrug-resistant gram-negative bacilli that can cause infections associated with high case fatality rates, and are emerging as epidemiologically important health care–associated pathogens in the United States (1). Prevention of CRE transmission in health care settings is dependent on recognition of cases, isolation of colonized and infected patients, effective use of infection control measures, and the correct use of antibiotics. The use of molecular technologies, including polymerase chain reaction (PCR) testing, pulsed-field gel electrophoresis (PFGE), and whole genome sequencing (WGS), can lead to detection of transmission events and interruption of transmission. In Wisconsin, acute care and critical access hospitals report laboratory-identified CRE to the Wisconsin Division of Public Health (WDPH), and clinical laboratories submit CRE isolates to the Wisconsin State Laboratory of Hygiene (WSLH) for molecular testing. During February–May 2015, a total of 49 CRE isolates from 46 patients were submitted to WSLH. On June 8, WSLH informed WDPH of five carbapenemase-producing CRE isolates with closely related PFGE patterns identified among four inpatients at two hospitals in southeastern Wisconsin. An investigation revealed a high degree of genetic relatedness among the patients’ isolates, but did not identify the mechanism of transmission between the two facilities. No breaches in recommended practices were identified; after reviewing respiratory care procedures, no further cases were identified. Routine hospital- and laboratory-based surveillance can detect and prevent health care transmission of CRE.
Since December 1, 2011, WDPH, under its authority in the Department of Health Services Administrative Code Chapter 145, has required all 138 Wisconsin acute care and critical access hospitals to report laboratory-identified CRE, using the multidrug-resistant organism and Clostridium difficile infection module of the National Healthcare Safety Network (2). The WSLH laboratory-based CRE surveillance program requests all clinical microbiology laboratories to submit carbapenem-nonsusceptible Enterobacteriaceae isolates to WSLH for PCR testing to determine the presence of genes encoding carbapenemase, including KPC, NDM, IMP, VIM, and OXA-48. All CRE isolates determined by PCR testing to have a carbapenemase gene are subtyped by PFGE testing to detect clusters; CRE isolates with PFGE patterns that are indistinguishable or closely related (1–2 band difference) are reported to WDPH’s health care-associated–infection prevention program for epidemiologic follow-up. WSLH’s use of WGS to detect single nucleotide polymorphisms (SNPs) of enteric bacterial pathogens and subsequent expansion of WGS to nonenteric bacteria has further enhanced the capability of WSLH to make genetic comparisons of CRE isolates of interest (3).
During February–May 2015, a total of 49 CRE isolates from 46 patients that met the National Healthcare Safety Network case definition for laboratory-identified CRE events (2) were submitted to WSLH (Figure 1). On June 8, 2015, WSLH notified WDPH that five carbapenemase-producing CRE isolates with closely related PFGE patterns had been identified among four inpatients at two hospitals in southeastern Wisconsin. A subsequent investigation included analysis of routine PFGE subtyping to detect clusters among all carbapenemase-producing CRE isolates submitted to WSLH and identify possible transmission events not recognized by hospital personnel. WSLH performed WGS on the five-cluster KPC-CRE isolates to characterize further the genetic relatedness. Interpretation of WGS was done at CDC using Lyve-SET, analysis software that identifies high quality SNPs (hqSNPs; sites with at least 10X coverage and 75% consensus)* (4). The bootstrap statistical method (resampling with replacement) was used to assess phylogenetic variation among genes in the WGS.
To determine hospital care points common to the four patients and possible modes of CRE transmission, WDPH personnel developed an instrument for epidemiologic data collection and conducted medical record reviews, site visits (October 28 and November 9, 2015), a review of respiratory care protocols, and interviews with infection prevention staff members, primary care providers, and patients (when available). During July 15–August 12, 2015, active surveillance was conducted in the respiratory units of concern at the two hospitals to determine whether ongoing transmission of KPC-CRE was occurring. Surveillance rectal swabs were collected once weekly among all patients hospitalized in the two respiratory units and submitted to WSLH for CRE culture.
Among the 49 isolates submitted during February–May 2015 (Figure 1), one cluster of five KPC-CRE isolates with two closely related PFGE patterns was detected among four inpatients (patients A–D) at two hospitals (hospital 1 and hospital 2) in southeastern Wisconsin: one isolate each from patients A, B, and D, and two isolates from patient C (Figure 2). The remaining 44 isolates, which included 20 KPC-CRE isolates, had unique PFGE patterns that did not match one another or the cluster patterns.
Isolates obtained from patients A and B (hospital 1) differed by two hqSNPs; no hqSNP differences were detected among isolates from patients C and D (hospital 2). Isolates from patients A and B each differed from isolates from patients C and D by only one hqSNP (Figure 3), indicating a high degree of sequence relatedness among all five KPC-CRE isolates. This is consistent with the occurrence of one or more intrafacility transmission events in hospital 1 and hospital 2.
Median age of the four patients was 65 years (range = 52–75 years), all were non-Hispanic whites, and two were women; median hospitalization length was 83 days (range = 65–103 days). Illnesses diagnosed among the patients at admission included postviral ascending weakness consistent with Guillain-Barré syndrome, cerebrovascular accident, pneumonia, and bacteremia during and after chemotherapy, radiation, and surgical resection of a glioblastoma. All four patients had been intubated and undergone a tracheostomy and had previous percutaneous endoscopic gastrostomy performed. However, none of these procedures had occurred at the same facility. None of the patients had undergone a gastrointestinal procedure that placed them at high risk for exposure to CRE (e.g., endoscopic retrograde cholangiopancreatography) (5).
Patient A was hospitalized during December 11, 2014–January 22, 2015, and patient B was hospitalized during March 20–April 26, 2015, in the same respiratory unit of hospital 1, but 57 days apart. Patient C was hospitalized during March 2–May 8, 2015, in hospital 2’s medical respiratory intensive care unit, and patient D was hospitalized during February 26–April 6, 2015, on the orthopedic surgical floor and was subsequently hospitalized in the medical respiratory intensive care unit during June 2–June 16, 2015, 25 days after patient C was discharged. On March 22, 2015, patients C and D had a 24-hour period of overlap in hospital 2’s medical respiratory intensive care unit, when patient D was moved from the orthopedic surgical floor for acute respiratory management. Although patients A–D were not transferred between hospitals 1 and 2, patient transfers between these two facilities are common.
A total of 122 rectal swabs were collected among 83 patients hospitalized in the two respiratory units during July 15–August 12 (the active surveillance period). During this period, a patient with previously known KPC-CRE infection (Patient E) was transferred from hospital 2 to hospital 1. Other than a specimen isolate from patient E, which did not match the cluster isolates, no KPC-CRE isolates were recovered in culture and no evidence of further CRE transmission was detected.
WDPH personnel conducted site visits and reviewed infection prevention protocols and policies for care of the ventilator circuit with infection prevention personnel at both facilities. These reviews were based on CDC Guidelines for Preventing Health-Care–Associated Pneumonia (6). No breaches in recommended practices were identified; however, infection prevention personnel could not describe respiratory personnel hand hygiene practices after handling of the circuit tubing. Thus, WDPH personnel recommended a facility compliance check of those practices. Public health actions to prevent future transmission of CRE at these hospitals included WDPH personnel working with infection prevention staff members regarding infection prevention measures related to ventilator care. No subsequent clusters of KPC-CREs have been reported from hospitals 1 and 2.
Discussion
Although the precise mechanism of CRE transmission was not determined, WDPH personnel used the detection of the KPC-CRE cluster to raise awareness among the hospitals’ infection prevention staff members regarding the possibility of intrafacility CRE transmission events among their patients. The circumstances provided an opportunity for review of facility infection prevention practices and respiratory care processes critical to prevention of health care–associated pneumonia. After addressing these concerns, no evidence of further transmission of these closely related strains of KPC-CRE at these facilities was found.
The investigation demonstrated the importance of routine hospital- and laboratory-based surveillance for the detection of health care–related transmission of CRE. The use of molecular subtyping methods (e.g., PFGE and WGS) to determine genetic relatedness of the bacterial isolates was particularly valuable. Matching PFGE patterns among isolates and subsequent WGS analysis of KPC-CRE led to focused epidemiologic investigations, subsequent cluster identification, and opportunities to provide infection prevention education to staff members at the involved hospitals.
Although routine use of PFGE and subsequent WGS in this investigation represents a novel application of technology to detect CRE transmission, the burden of resources might preclude similar use in states with medium-to-high prevalences of CRE. However, the increasing availability of WGS might improve utility of this approach in the future. In Wisconsin, a state with relatively low CRE prevalence since the inception of statewide CRE surveillance during December 2011–April 2016, PFGE has been conducted on 225 CRE isolates (average = ~50 per year). Five clusters have been detected, and attendant public health–related responses likely prevented further transmission and case occurrences in health care facilities.
This report is subject to at least one limitation. PFGE patterns can be remarkably similar among certain CRE in the absence of any epidemiologic link. This is especially true of ST258 CR-producing Klebsiella pneumoniae (7). Therefore, PFGE data must be considered with epidemiologic data to determine potential transmission events.
Multidrug-resistant organisms, in particular CRE, have the capability of spreading undetected, with the possibility of devastating outbreaks in health care settings (8). Routine hospital- and laboratory-based surveillance for the detection of CRE and the use of molecular techniques to characterize isolates can detect and reduce occurrence of multidrug-resistant infections through interventions designed to interrupt transmission. Timely access to technology and results can facilitate rapid implementation of effective interventions (9).
Acknowledgments
Infection prevention, nursing, and respiratory staff members from hospital 1 and hospital 2, southeastern Wisconsin.
Corresponding author: Lina I. Elbadawi, lelbadawi@cdc.gov, 608-266-0392.
1Epidemic Intelligence Service, Division of Scientific Education and Professional Development, CDC; 2Bureau of Communicable Diseases, Wisconsin Division of Public Health; 3Wisconsin State Laboratory of Hygiene, 4Division of Healthcare Quality Promotion, CDC.
References
- CDC. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep 2013;62:165–70. PubMed
- CDC. Multidrug-resistant organism & Clostridium difficile infection (MDRO/CDI) module. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. http://www.cdc.gov/nhsn/pdfs/pscmanual/12pscmdro_cdadcurrent.pdf
- Marsh JW, Krauland MG, Nelson JS, et al. Genomic epidemiology of an endoscope-associated outbreak of Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae. PLoS One 2015;10:e0144310. CrossRef PubMed
- Katz LS, Petkau A, Beaulaurier J, et al. Evolutionary dynamics of Vibrio cholerae O1 following a single-source introduction to Haiti. MBio 2013;4:e00398–13. CrossRef PubMed
- Epstein L, Hunter JC, Arwady MA, et al. New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA 2014;312:1447–55. CrossRef PubMed
- Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R; CDC; Healthcare Infection Control Practices Advisory Committee. Guidelines for preventing health-care–associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53(No. RR-3). PubMed
- Navon-Venezia S, Leavitt A, Schwaber MJ, et al. ; Israeli KPC Kpn Study Group. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009;53:818–20. CrossRef PubMed
- Bush K. Bench-to-bedside review: the role of beta-lactamases in antibiotic-resistant Gram-negative infections. Crit Care 2010;14:224. CrossRef PubMed
- Ben-David D, Maor Y, Keller N, et al. Potential role of active surveillance in the control of a hospital-wide outbreak of carbapenem-resistant Klebsiella pneumoniae infection. Infect Control Hosp Epidemiol 2010;31:620–6. CrossRef PubMed
* 10X coverage means each position must have at least 10 Illumina reads map to it; 75% consensus means that the identity of each position must be ≥75% of a single nucleotide (https://github.com/lskatz/lyve-SET).
 FIGURE 1. Number of laboratory-confirmed carbapenem-resistant Enterobacteriaceae (CRE) isolates,* by date of specimen collection — Wisconsin, February–May 2015
FIGURE 1. Number of laboratory-confirmed carbapenem-resistant Enterobacteriaceae (CRE) isolates,* by date of specimen collection — Wisconsin, February–May 2015
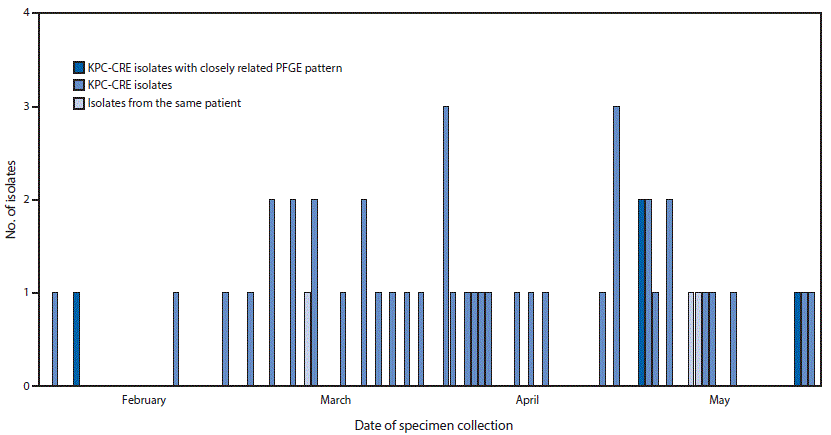
Abbreviation: PFGE = pulsed-field gel electrophoresis.
* N = 49 isolates from 46 unique patients.
 FIGURE 2. Pulsed-field gel electrophoresis (PFGE) subtyping comparison of five KPC-producing Klebsiella pneumoniae isolates digested with XbaI — Wisconsin, February–May 2015
FIGURE 2. Pulsed-field gel electrophoresis (PFGE) subtyping comparison of five KPC-producing Klebsiella pneumoniae isolates digested with XbaI — Wisconsin, February–May 2015
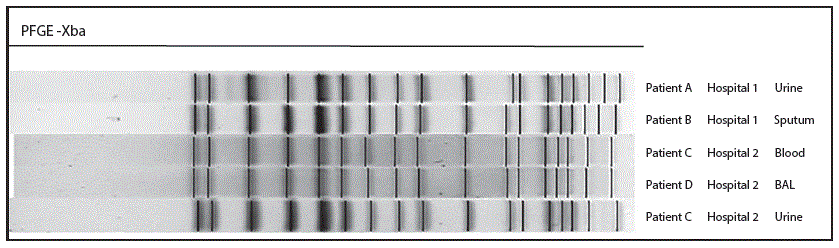
Abbreviation: BAL = bronchoalveolar lavage.
 FIGURE 3. Maximum likelihood phylogenetic tree* of five carbapenem-resistant Enterobacteriaceae isolates from four patients — Wisconsin, February–May 2015†,§
FIGURE 3. Maximum likelihood phylogenetic tree* of five carbapenem-resistant Enterobacteriaceae isolates from four patients — Wisconsin, February–May 2015†,§
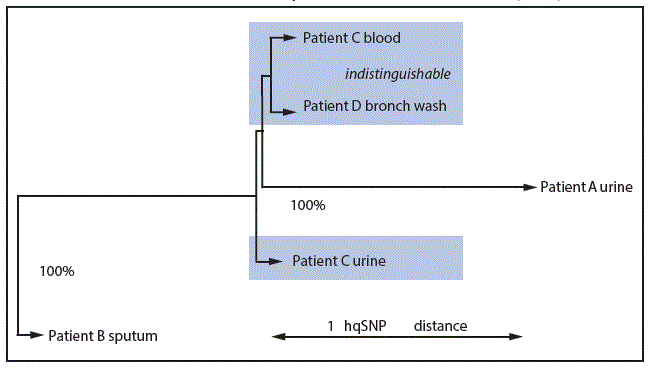
Abbreviation: bronch = bronchoalveolar; hqSNP = high quality single nucleotide polymorphism.
* 100 bootstraps performed; bootstraps with <50% confidence are not labeled at their nodes.
† The three isolates in shaded areas were indistinguishable from one another (i.e., 0 hqSNPs apart).
§ To focus on the five outbreak leaves, the two unrelated control isolates used to root the phylogeny are not illustrated.
Suggested citation for this article: Elbadawi LI, Borlaug G, Gundlach KM, et al. Carbapenem-Resistant Enterobacteriaceae Transmission in Health Care Facilities — Wisconsin, February–May 2015. MMWR Morb Mortal Wkly Rep 2016;65:906–909. DOI: http://dx.doi.org/10.15585/mmwr.mm6534a5.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: August 17, 2017
- Page last updated: August 17, 2017
- Content source:


 ShareCompartir
ShareCompartir