Update on Vaccine-Derived Polioviruses — Worldwide, January 2015–May 2016
Weekly / August 5, 2016 / 65(30);763–769
Jaume Jorba, PhD1; Ousmane M. Diop, PhD2; Jane Iber, MSc1; Roland W. Sutter, MD3; Steven G. Wassilak, MD4; Cara C. Burns, PhD1 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Vaccine-derived polioviruses (VDPVs), genetically divergent strains from the oral poliovirus vaccine (OPV), fall into three classifications: 1) circulating VDPVs (cVDPVs) from outbreaks, 2) immunodeficiency-associated VDPVs (iVDPVs) from patients with primary immunodeficiencies, and 3) ambiguous VDPVs (aVDPVs) that cannot be more definitively identified. cVDPVs emerge in settings of low population immunity, can cause paralysis, and can sustain long-term circulation. Because >94% of cVDPVs isolated since 2006 and 66% of iVDPVs identified since OPV introduction are type 2, and because wild polio virus type 2 was declared eradicated in 2015, the World Health Organization coordinated worldwide replacement of trivalent OPV with bivalent OPV (types 1 and 3) in April 2016.
What is added by this report?
During January 2015–May 2016, new cVDPV outbreaks were identified in Burma, Guinea, Laos, Madagascar, and Ukraine, while cVDPV2 circulation in Nigeria and Pakistan sharply declined. Twenty-one newly identified persons in 10 countries were found to excrete iVDPVs.
What are the implications for public health practice?
The ultimate goal of the Global Polio Eradication Initiative is the cessation of all poliovirus circulation. The risk for iVDPV emergence will continue as long as OPV is used. The switch from trivalent OPV to bivalent OPV in April 2016 was the first step to phasing out the use of all OPV, setting the stage for a total worldwide shift from OPV to IPV.
In 1988, the World Health Assembly resolved to eradicate poliomyelitis worldwide (1). One of the main tools used in polio eradication efforts has been the live, attenuated, oral poliovirus vaccine (OPV) (2), an inexpensive vaccine easily administered by trained volunteers. OPV might require several doses to induce immunity, but provides long-term protection against paralytic disease. Through effective use of OPV, the Global Polio Eradication Initiative (GPEI) has brought wild polioviruses to the threshold of eradication (1). However, OPV use, particularly in areas with low routine vaccination coverage, is associated with the emergence of genetically divergent vaccine-derived polioviruses (VDPVs) whose genetic drift from the parental OPV strains indicates prolonged replication or circulation (3). VDPVs can emerge among immunologically normal vaccine recipients and their contacts as well as among persons with primary immunodeficiencies (PIDs). Immunodeficiency-associated VDPVs (iVDPVs) can replicate for years in some persons with PIDs. In addition, circulating vaccine-derived polioviruses (cVDPVs) (3) can emerge in areas with low OPV coverage and can cause outbreaks of paralytic polio. This report updates previous summaries regarding VDPVs (4).
During January 2015–May 2016, five new cVDPV outbreaks were identified in Burma (Myanmar) (two cases), Guinea (seven cases), Laos (11 cases), Madagascar (10 cases), and Ukraine (two cases) (5), while cVDPV type 2 (cVDPV2) circulation in Nigeria and Pakistan decreased sharply. Twenty-one newly identified persons in 10 countries were found to excrete iVDPVs, and a patient in the United Kingdom was still excreting an iVDPV in 2015 after >29 years of chronic infection. Ambiguous VDPVs (aVDPVs), isolates that cannot be classified definitively, were found among immunocompetent persons and environmental samples in 19 countries.
Global eradication of wild poliovirus type 2 was declared in September 2015, and wild poliovirus type 3 has not been detected since 2012. Currently, wild poliovirus type 1 transmission has been identified only in Afghanistan and Pakistan. Because the majority of VDPV isolates that have emerged from OPV use in recent years are type 2, the World Health Organization coordinated the worldwide replacement of trivalent OPV (tOPV; Sabin types 1, 2, and 3) with bivalent OPV (bOPV; types 1 and 3) in April 2016, preceded by introduction of at least 1 dose of injectable inactivated poliovirus vaccine (IPV) into routine immunization schedules in countries with higher risk for VDPV2 emergence and spread (6).
Properties of VDPVs
VDPVs are polioviruses whose genetic divergence from the parental OPV strains indicates prolonged replication or circulation (3). Three poliovirus serotypes (PV1, PV2, and PV3) have been identified. Poliovirus isolates are grouped into three categories: wild polioviruses (WPVs), vaccine-related polioviruses (VRPVs), and vaccine-derived polioviruses (VDPV). WPVs are capable of sustained person-to-person transmission without genetic evidence of vaccine strain origin. VRPVs have limited divergence (<1% divergent [PV1 and PV3] or <0.6% divergent [PV2]) in the VP1 nucleotide sequences from the corresponding OPV strain. VDPVs are >1% divergent (for PV1 and PV3) or >0.6% divergent (for PV2) in VP1 sequences from the corresponding OPV strain (3). VDPVs are further classified as 1) cVDPVs, when evidence of person-to-person transmission in the community exists; 2) iVDPVs, when they are isolated from persons with PIDs; and 3) aVDPVs, when they are clinical isolates from persons with no known immunodeficiency and no evidence of transmission, or they are sewage isolates that are unrelated to other known VDPVs and whose source is unknown (3).
Virologic Testing for VDPVs
All poliovirus isolates are characterized by laboratories of the Global Polio Laboratory Network (4). VDPV screening is conducted using real-time reverse transcription–polymerase chain reaction (rRT-PCR) nucleic acid amplification, targeted to nucleotide substitutions that frequently revert to the parental WPV sequence during replication of OPV in the human intestine (7). Potential VDPVs identified by rRT-PCR screening are sequenced in the VP1 region for definitive analysis; the complete genome is sequenced if required for higher-resolution analysis.
Detection of cVDPVs
During January 2015–May 2016, the number of countries with detected cVDPV circulation increased from four to seven (Figure 1) (4). Outbreaks in South Sudan (cVDPV2) and Afghanistan (cVDPV2) appear to have been interrupted. Outbreaks of cVDPV2 in Pakistan and Nigeria have declined to very low incidence levels (4,8). New outbreaks were reported in Ukraine (cVDPV type 1 [cVDPV1], two cases), Burma (cVDPV2, two cases), Guinea (cVDPV2, seven cases), Laos (cVDPV1, 11 cases), and Madagascar (cVDPV1, 10 cases) (Table). Among the 721 cVDPV cases detected worldwide during January 2006–May 2016, 681 (94%) were associated with cVDPV2, and 31 (4%) were associated with cVDPV1; however, during January 2015–May 2016, among 35 cVDPV cases, 23 (66%) were cVDPV1 (Table) (Figure 2).
Guinea. During 2015, seven cVDPV2s were isolated from patients aged <15 years with acute flaccid paralysis (AFP) in Kankan Province (up to 3% VP1 divergence). The first detected cVDPV2 associated with this outbreak was isolated from a patient in the same province with an August 2014 paralysis onset date.
Laos. Eight cVDPV1 cases in 2015 and three cases in 2016 were detected in three adjacent provinces (2.3%–3.9% VP1 divergence). The most recent case was reported in Fuang District of Vientiane Province, with paralysis onset in January 2016.
Madagascar. A cVDPV1 outbreak was initially detected in September 2014 in Analalava, Mahjanga Province, on the northwest coast; the virus circulated widely throughout the country during 2015. Genetically linked viruses were isolated in 2015; 10 AFP cases and 11 isolates were identified through community-based surveillance, with VP1 nucleotide sequence divergence up to 3.3% from the parental OPV strain.
Burma (Myanmar). During April and October 2015, two related cVDPV2s (1.4%–1.7% VP1 divergence) were detected from two AFP cases in the same province; the most recent isolate was from an AFP case in Rakhine province with onset date October 5, 2015.
Nigeria. Low-level circulation in northern states continued during January 2015–May 2016 (4). Virus from the major cVDPV2 lineage group that first emerged in 2005 (8) was isolated from a sewage sample collected on March 4, 2015 (7.3% VP1 divergence). Virus from an independent cVDPV2 emergence (3.5% VP1 divergence from Sabin 2 and 2.2% divergence from its nearest relative), originating in Chad in 2012 (9), was isolated from sewage samples; the most recent positive sample was reported from Borno State on April 29, 2016 (10). In addition, one Kaduna State sewage isolate and an isolate from an AFP case were linked to the outbreak detected in 2014 (the most recent positive sample was reported on May 28, 2015 [1.4% VP1 divergence]) (4).
Pakistan. Among the five independent cVDPV2 emergences reported previously (4), only one persisted during January 2015–May 2016, detected in 14 environmental samples collected in Sindh and one in Baluchistan. Two AFP cases reported in Federally Administered Tribal Areas and Khyber Pakhtunkhwa with onset in February 2015 were genetically linked to a new cVDPV2 emergence (0.7% divergent from parental Sabin 2). This new cVDPV2 emergence was not detected after February 2015. No cVDPVs have been detected in 2016.
Ukraine. In 2015, two genetically linked cVDPV1s (2.2%–2.9% VP1 divergence) were detected in southwestern Ukraine, from two AFP cases with onset dates of June 30 and July 7.
Detection of iVDPVs
After implementation of intensified surveillance for iVDPVs, detection of new iVDPV infections increased from eight in 2014 to 21 during January 2015–May 2016. (Table). During this reporting period, with the exception of two type 3 iVDPVs, all were type 2. Like cVDPVs, the cumulative serotype distribution since OPV introduction shows that type 2 iVDPVs are the most prevalent (66%), followed by type 1 (14%), type 3 (14%), and heterotypic mixtures (6%). Selected iVDPVs from the reporting period are described below.
Egypt. A male child aged 11 months with PID developed paralysis in December 2015; iVDPV2 was detected. In April 2016, an unrelated iVDPV2 was isolated from a nonparalyzed PID patient.
Iran. During this reporting period, five patients (one with AFP) were found to be excreting iVDPVs. A girl aged 6 months with severe combined immunodeficiency (SCID), who received OPV in March 2015, developed AFP in September 2015. The last positive sample from this child was in February 2016. Four nonparalytic SCID patients were found to be excreting type 2 iVDPVs; two of these patients (one each from Tehran and Ardebil provinces) died; the other two were from Golestan and Kermanshah provinces.
Iraq. A girl with PID developed AFP at age 8 months. In July 2015, iVDPV2 was detected, and the girl subsequently died.
Oman. A boy with major histocompatibility complex class II deficiency was found to be infected with iVDPV2 at age 9 months.
West Bank and Gaza. In October 2015, a girl aged 5 months with SCID who had not developed AFP was found to be infected with an iVDPV2. She remains hospitalized after bone marrow transplantation and continues to excrete iVDPV2.
Detection of aVDPVs
During January 2015–May 2016, aVDPVs were isolated in 19 countries (Table). The most divergent aVDPV (3.9% VP1 divergence) was isolated from an AFP case in Madagascar. This represented an emergence independent from a cVDPV emergence that circulated broadly in Madagascar during the same period. Report of aVDPVs in settings with immunization coverage <60% might indicate a risk for cVDPV emergence and further spread and potential gaps in surveillance. Selected aVDPVs from the reporting period are described below.
Chad. An aVDPV2 (0.8% VP1 divergence) was isolated from an AFP case with paralysis onset in January 2015 in Mayo-Kebbi Est Province.
Democratic Republic of the Congo. Four independent aVDPV2s were isolated from four AFP clinical samples: two in 2015 (0.8%–1.1% VP1 divergence) and two in 2016 (0.7%–1.7% VP1 divergence). The latest isolate from 2016 resembles an iVDPV2, but because no immunodeficient source patient has been identified, classification of this VDPV is pending.
Egypt. Four environmental samples contained aVDPVs (0.7–0.9% VP1 divergence), three in 2015 and one in 2016. They were collected from four distinct collection sites during February 2015–March 2016.
Kenya. An aVDPV2 (0.8% VP1 divergence) was isolated from a sewage specimen collected in Nairobi in December 2015. The virus had four amino acid differences from Sabin 2, all in the neutralizing antigenic sites, suggesting an iVDPV. However, no immunodeficient source patient has been identified.
Madagascar. An aVDPV1 (3.9% VP1 divergence) was isolated from a patient in Nosy-Varika, Fianarantsoa Province, on the central east coast, who had AFP onset on January 31, 2015. Despite a small number of VP1 substitutions shared with the 2014 cVDPV1 isolates from Analalava, on the northwest coast, the sequence properties of this aVDPV1 are consistent with an independent VDPV1 emergence. Thus, two emergences of VDPV were detected, but only one sustained circulation.
Netherlands. An aVDPV3 was isolated from a non-AFP case in a Syrian refugee who arrived in Netherlands in 2014. The date of the last positive specimen (1.7% VP1 divergence) was June 16, 2015.
Nigeria. Four aVDPV2s (all from sewage samples; all with 0.7%–0.8% VP1 divergence) were isolated in Sokoto State during the reporting period; the most recent sample was collected on March 9, 2015. Three of the isolates were genetically linked, although closely related (within 2 nucleotide differences), and detection was limited to two serial collections, on February 9 and March 9. An aVDPV2 was isolated from an AFP patient who developed paralysis on May 14, 2016, in Jigawa State.
Pakistan. Ten aVDPV2s (two from AFP patients and eight from sewage samples; 0.7%–1.2% VP1 divergence) were isolated in 2015. The most recent aVDPV2 isolates were from an AFP patient in Sindh province in August 2015 (1.0% VP1 divergence), and from a sewage sample collected in Baluchistan in December 2015 (0.7% VP1 divergence).
Discussion
The number of cVDPV outbreaks worldwide increased since the January 2014–March 2015 reporting period; however, the intensity and number of AFP cases in cVDPV outbreaks declined. Inclusion of more tOPV rounds in the steadily improving supplementary immunization activities (SIAs)* and increased access to unimmunized children were important factors for interruption of cVDPV2 outbreaks in Afghanistan and South Sudan and for control of cVDPV2 outbreaks in Nigeria and Pakistan. The new outbreaks in Burma, Guinea, Laos, Madagascar, and Ukraine highlight the importance of maintaining high population immunity to all polioviruses, as well as sensitive AFP surveillance.
The expansion of environmental surveillance in countries at high risk has increased the sensitivity of poliovirus detection. However, detection of polioviruses from sewage presents logistical and technical challenges (4), including determination of VDPV genetic signatures (7). Determination of epidemiologic linkages from sequence data in environmental isolates represents an additional challenge. For example, highly divergent isolates, most likely representing iVDPVs based on the genetic signature, are classified as aVDPVs because of the absence of epidemiologic linkage to a known immunodeficient patient who is a chronic poliovirus excretor.
The frequency of cVDPV2 detection declined during January 2015–May 2016. However, the emergence of cVDPV2 in countries with low routine vaccination coverage underscores the risks from widening immunization gaps to type 2 polioviruses. The April 29, 2016, report of detection of a cVDPV2 isolate from sewage in Nigeria with 3.5% VP1 divergence suggests that gaps in surveillance had missed virus circulation. In response to this outbreak, three rounds of SIAs with monovalent oral poliovirus vaccine type 2 (mOPV2) were used in accessible areas of Borno State and neighboring districts in two other states (10). Detection of aVDPV2 isolates in environmental samples in Kenya and Egypt with six or seven VP1 nucleotide differences (<1 year of replication/circulation) did not lead to a recommendation for use of mOPV2; scope of response is based on risk of spread and the estimated duration of circulation before detection.
WPV2, which has not been detected since 1999, was declared globally eradicated on September 20, 2015, and WPV3 has not been detected worldwide since 2012. A key goal of the polio endgame strategic plan (6) is the global cessation of all OPV use, starting with OPV2, which will ultimately eliminate the risk for cVDPV outbreaks and new iVDPV infections. During a 2-week period in April 2016, the Global Polio Eradication Initiative coordinated worldwide withdrawal of tOPV (types 1, 2, and 3) and replacement with bOPV (types 1 and 3), which was accomplished by May 2016 in 150 countries and territories that used any OPV in childhood vaccination schedules. The Global Polio Eradication Initiative and Global Polio Laboratory Network have continued to strengthen AFP and poliovirus surveillance during 2016. Routine immunization services also are being strengthened, and most countries incorporated at least 1 dose of IPV into routine childhood immunization schedules in 2015 (6). This was limited from the planned introduction in all 126 countries that used OPV exclusively for routine immunization because of a global IPV supply shortage. To reduce the risk for iVDPV spread from long-term chronic excretors, maintenance of high levels of routine vaccination coverage will be necessary during the polio endgame.
Acknowledgments
Global Polio Laboratory Network; Humayun Asghar, MD, World Health Organization (WHO) Regional Office, Cairo, Egypt; Eugene Gavrilin, PhD, WHO Regional Office, Copenhagen, Denmark; Nicksy Gumede, PhD, WHO Regional Office, Brazzaville, Republic of Congo; Grace Macklin, PhD, Fem Paladin, PhD, Merja Roivainen, PhD, Graham Tallis, PhD, WHO, Geneva, Switzerland; Gloria Rey, MSc, WHO Regional Office, Washington, DC; Sirima Pattamadilok, MSc, WHO Regional Office, New Delhi; W. William Schluter, MD, Yan Zhang, PhD, WHO Regional Office, Manila, Philippines; Deepa Sharma, PhD, Jagadish Deshpande, PhD, Enterovirus Research Center, Indian Council of Medical Research, Mumbai, India; Shohreh Shahmahmoodi, PhD, Tehran University of Medical Sciences, Tehran, Iran; Salmaan Sharif, PhD, Sohail Zaidi, MSc, NIH Pakistan, Islamabad, Pakistan; Merav Weil, PhD, Lester Shulman, PhD, Central Virology Laboratory, Tel Hashomer, Israel; Henda Triki PhD, Insitut Pasteur, Tunis, Tunisia; Yong Zhang, PhD, China CDC, Beijing, China; Eric Mast, MD, Global Immunization Division, Center for Global Health, CDC; Qi Chen, MSc, Hongmei Liu, MSc, M. Steven Oberste, PhD, Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC.
Corresponding author: Cara C. Burns, cburns@cdc.gov, 404-639-5499.
1Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC; 2Department of Polio Eradication, Detection and Interruption Unit, World Health Organization, Geneva, Switzerland; 3Department of Polio Eradication, Research, Policy and Containment Unit, World Health Organization, Geneva, Switzerland; 4Global Immunization Division, Center for Global Health, CDC.
References
- Morales M, Tangermann RH, Wassilak SG. Progress toward polio eradication—worldwide, 2015–2016. MMWR Morb Mortal Wkly Rep 2016;65:470–3. CrossRef PubMed
- Sutter RW, Kew OM, Cochi SL, Aylward RB. Poliovirus vaccine—live. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 6th ed. London, UK: W.B. Saunders; 2013:598–645.
- Burns CC, Diop OM, Sutter RW, Kew OM. Vaccine-derived polioviruses. J Infect Dis 2014;210(Suppl 1):S283–93. CrossRef PubMed
- Diop OM, Burns CC, Sutter RW, Wassilak SG, Kew OM. Update on vaccine-derived polioviruses—worldwide, January 2014–March 2015. MMWR Morb Mortal Wkly Rep 2015;64:640–6. PubMed
- Morales M, Nnadi CD, Tangermann RH, Wassilak SG. Notes from the field: circulating vaccine-derived poliovirus outbreaks—five countries, 2014–2015. MMWR Morb Mortal Wkly Rep 2016;65:128–9. CrossRef PubMed
- Global Polio Eradication Initiative. Polio eradication and endgame strategic plan (2013–2018). Geneva, Switzerland: World Health Organization, Global Polio Eradication Initiative; 2013. http://www.polioeradication.org/portals/0/document/resources/strategywork/endgamestratplan_20130414_eng.pdf
- Kilpatrick DR, Ching K, Iber J, et al. Identification of vaccine-derived polioviruses using dual-stage real-time RT-PCR. J Virol Methods 2014;197:25–8. CrossRef PubMed
- Burns CC, Shaw J, Jorba J, et al. Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in northern Nigeria. J Virol 2013;87:4907–22. CrossRef PubMed
- Diop OM, Burns CC, Wassilak SG, Kew OM. Update on vaccine-derived polioviruses – worldwide, July 2012-December 2013. MMWR Morb Mortal Wkly Rep 2014;63:242–8. PubMed
- Etsano A, Damisa E, Shuaib F, et al. Environmental isolation of circulating vaccine-derived poliovirus after interruption of wild poliovirus transmission—Nigeria, 2016. MMWR Morb Mortal Wkly Rep 2016;65:770–3.
* Supplementary immunization activities are mass vaccination campaigns conducted over a short period (days to weeks) during which a dose of OPV is administered to all children aged <5 years, regardless of previous vaccination history. Campaigns can be conducted nationally or in selected areas of a country.
 FIGURE 1. Vaccine-derived polioviruses (VDPVs) detected, by serotype and VDPV classification — worldwide, January 2015–May 2016*
FIGURE 1. Vaccine-derived polioviruses (VDPVs) detected, by serotype and VDPV classification — worldwide, January 2015–May 2016*
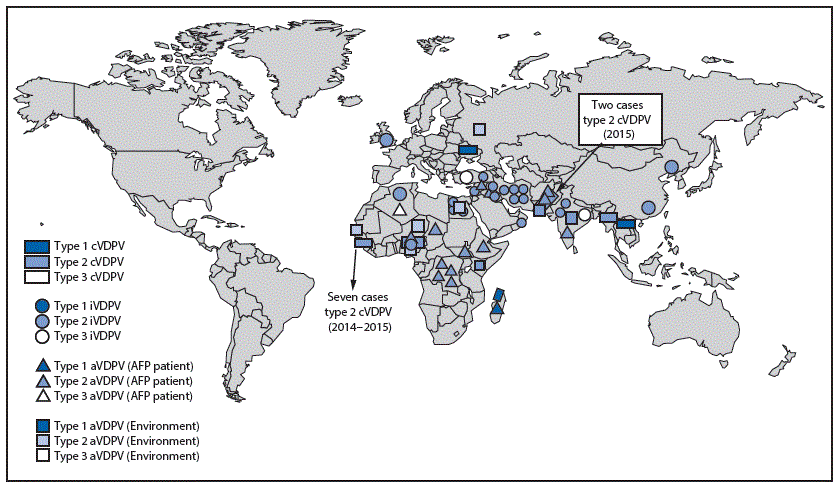
Abbreviations: cVDPV = circulating VDPV; iVDPV = immunodeficiency-associated VDPV; aVDPV = ambiguous VDPV; AFP = acute flaccid paralysis.
* Spread of cVDPVs followed the elimination of the corresponding serotype of indigenous wild poliovirus, but with continued introduction of oral poliovirus vaccine into communities with growing immunity gaps. All of the cVDPV outbreaks were detected first by the laboratory, using sequence data and evolutionary analyses.
 TABLE. Number of vaccine-derived polioviruses (VDPVs) detected, by classification and other selected characteristics — worldwide, January 2015–May 2016
TABLE. Number of vaccine-derived polioviruses (VDPVs) detected, by classification and other selected characteristics — worldwide, January 2015–May 2016
| Country | Year(s) detected* | Source† | Serotype | No. of isolates§ January 2015–May 2016 | VP1 divergence from Sabin OPV strain (%)¶ | Routine OPV3 coverage (%)** | Estimated duration of VDPV replication†† | Current status (date of last outbreak case, patient isolate, or environmental sample) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases | No. of contacts | No. of non-AFP sources | ||||||||
| Circulating VDPVs (cVDPVs) | ||||||||||
| Guinea | 2014–15 | Outbreak | 2 | 7 | 6 | 0 | 2.4–3.0 | 42 | 2.7 yrs | Dec 26, 2015 |
| Laos | 2015–16 | Outbreak | 1 | 11 | 25 | 0 | 2.3–3.9 | 88 | 3.5 yrs | Jan 11, 2016 |
| Madagascar | 2014–15 | Outbreak | 1 | 10 | 11 | 0 | 2.3–3.3 | 73 | 3 yrs | Sep 2, 2015 |
| Burma (Myanmar) | 2015 | Outbreak | 2 | 2 | 0 | 0 | 1.4–1.7 | 76 | 1.5 yrs | Oct 5, 2015 |
| Nigeria | 2005–15 | Outbreak | 2 | 0 | 0 | 1 | 7.3 | 72 | 6.6 yrs | Mar 4, 2015 |
| Nigeria | 2014–15 | Outbreak | 2 | 1 | 0 | 1 | 1.4 | 72 | ~1 yr | May 28, 2015 |
| Nigeria | 2013–16 | Outbreak – importation | 2 | 0 | 0 | 1 | 3.5 | 72 | ~3 yrs | Mar 23, 2016 |
| Pakistan | 2012–15 | Outbreak | 2 | 2 | 0 | 15 | 0.7–2.1 | 72 | ~2 yrs | Mar 28, 2015 |
| Ukraine | 2015 | Outbreak | 1 | 2 | 0 | 0 | 2.2–2.9 | 74 | 2.6 yrs | Jul 12, 2015 |
| Immunodeficiency-associated VDPVs (iVDPVs) | ||||||||||
| Algeria | 2015 | AFP patient | 2 | 1 | 0 | 0 | 1.7 | 95 | 1.5 yrs | Jul 22, 2015 |
| China | 2015 | AFP patient | 2 | 1 | 0 | 0 | 0.8 | 99 | <1 yr | Mar 12, 2015 |
| China | 2015 | AFP patient | 2 | 1 | 0 | 0 | 2.4 | 99 | ~2 yrs | Mar 19, 2015 |
| Egypt | 2015 | AFP patient PID | 2 | 1 | 0 | 0 | 1.9 | 94 | 1.7 yrs | Dec 9, 2015 |
| Egypt | 2016 | Non-AFP PID | 2 | 0 | 0 | 1 | 1.3 | 94 | ~1 yr | Apr 18, 2016 |
| Egypt | 2016 | Non-AFP PID | 2 | 0 | 0 | 1 | 2.0 | 94 | <2 yrs | May 22, 2016 |
| Nigeria | 2015 | AFP patient | 2 | 1 | 0 | 0 | 0.7 | 72 | <1 yr | Oct 9, 2015 |
| India | 2015 | AFP patient CVID | 2 | 1 | 0 | 0 | 2.7–4.0 | 82 | 2.3 yrs–4 yrs | Mar 8, 2016 |
| India | 2015 | AFP patient XLA | 2 | 1 | 0 | 0 | 0.7 | 82 | 7 mos | Feb 29, 2016 |
| India | 2015 | Non-AFP SCID | 3 | 0 | 0 | 1 | 4.5–10.2 | 82 | 3.9 yrs; 6 yrs | May 30, 2016 |
| Iran | 2015 | AFP patient SCID | 2 | 1 | 0 | 0 | 0.8–1.6 | 99 | ~1.5 yrs | Feb 7, 2016 |
| Iran | 2015 | Non-AFP SCID 1 | 2 | 0 | 0 | 1 | 1.3–1.8 | 99 | ~1.5 yrs | Feb 16, 2016 |
| Iran | 2015 | Non-AFP SCID 2 | 2 | 0 | 0 | 1 | 0.7 | 99 | <1 yr | Oct 14, 2015 |
| Iran | 2015 | Non-AFP SCID 3 | 2 | 0 | 0 | 1 | 1.1 | 99 | 1 yr | Oct 14, 2015 |
| Iran | 2015 | Non-AFP SCID 4 | 2 | 0 | 0 | 1 | 1.8–2.2 | 99 | 2 yrs | Feb 8, 2016 |
| Iraq | 2015 | AFP patient PID | 2 | 1 | 2 | 0 | 1.9 | 76 | 1.7 yrs | Jul 23, 2015 |
| Iraq | 2016 | AFP patient PID | 2 | 1 | 0 | 0 | 0.8 | 76 | <1 yr | Feb 13, 2016 |
| Oman | 2015 | Non-AFP PID | 2 | 0 | 0 | 1 | 0.8–1.6 | 99 | ~1.5 yrs | Nov 30, 2015 |
| Turkey | 2015 | Non-AFP PID | 3 | 0 | 0 | 1 | 1.7 | 96 | 1.5 yrs | Feb 22, 2015 |
| Turkey | 2015 | AFP patient PID | 2 | 1 | 0 | 0 | 0.7–0.8 | 96 | <1 yr | Mar 20, 2015 |
| UK | 2015 | Non-AFP PID | 2 | 0 | 0 | 1 | 16.6–16.7 | 96 | >29 yrs | Nov 17, 2015 |
| West Bank and Gaza Strip | 2015 | Non-AFP SCID | 2 | 0 | 0 | 1 | 1.0–1.9 | 96 | 1.7 yrs | May 3, 2016 |
| Ambiguous VDPVs (aVDPVs) | ||||||||||
| Algeria | 2015 | AFP patient | 3 | 1 | 0 | 0 | 1.6 | 95 | ~1.5 yrs | May 5, 2015 |
| Chad | 2015 | AFP patient | 2 | 1 | 0 | 0 | 0.8 | 54 | <1 yr | Jan 15, 2015 |
| China | 2015 | Non-AFP | 1 | 0 | 0 | 1 | 1.1 | 99 | 1 yr | Mar 20, 2015 |
| Democratic Republic of the Congo | 2015–16 | AFP patient | 2 | 4 | 0 | 0 | 0.7–1.8 | 79 | 1.5 yrs | Mar 29, 2016 |
| Egypt | 2015–16 | Environmental sample | 2 | 0 | 0 | 4 | 0.7–0.9 | 94 | <1 yr | Mar 15, 2015 |
| Ethiopia | 2015 | AFP patient | 2 | 1 | 0 | 0 | 0.8 | 75 | <1 yr | Mar 11, 2015 |
| India | 2015 | AFP patient | 2 | 1 | 0 | 0 | 0.8 | 82 | <1 yr | Mar 8, 2015 |
| India | 2015 | Environmental sample | 2 | 0 | 0 | 15 | 0.7–1.4 | 82 | 7 mos–1.3 yrs | May 16, 2016 |
| Iraq | 2015 | AFP patient | 2 | 1 | 0 | 0 | 1.0–1.3 | 76 | ~1 yr | Nov 24, 2015 |
| Kenya | 2015 | Environmental sample | 2 | 0 | 0 | 1 | 0.8 | 81 | <1 yr | Dec 30, 2015 |
| Madagascar | 2015 | AFP patient | 1 | 1 | 1 | 0 | 3.5–3.9 | 73 | 3.5 yrs | Feb 22, 2015 |
| Netherlands | 2015 | Non-AFP | 3 | 0 | 0 | 1 | 1.7 | 96 | 1.5 yrs | Jun 16, 2015 |
| Niger | 2015 | Environmental sample | 2 | 0 | 0 | 1 | 0.9 | 67 | <1 yr | Dec 29, 2015 |
| Nigeria | 2016 | AFP patient | 2 | 1 | 0 | 0 | 0.9 | 72 | <1 yr | May 18, 2016 |
| Nigeria | 2015 | Environmental sample | 2 | 0 | 0 | 4§§ | 0.7–0.8 | 72 | <1 yr | Mar 9, 2015 |
| Pakistan | 2015 | AFP patient | 2 | 2 | 0 | 0 | 1.0–1.2 | 72 | ~1 yr | Aug 20, 2015 |
| Pakistan | 2015 | Environmental sample | 2 | 0 | 0 | 8 | 0.7–1.0 | 72 | ~1 yr | Dec 12, 2015 |
| Russia | 2015 | Environmental sample | 2 | 0 | 0 | 1 | 17.6 | 97 | >15 yrs | Sep 17, 2015 |
| Republic of South Sudan | 2015 | AFP patient | 2 | 1 | 0 | 0 | 1.6 | 44 | ~1.5 yrs | Apr 22, 2015 |
| Senegal | 2015 | Environmental sample | 2 | 0 | 0 | 1 | 0.7 | 85 | <1 yr | Nov 5, 2015 |
| Syria | 2015 | AFP patient | 2 | 1 | 0 | 0 | 0.7 | 52 | <1 yr | May 13, 2016 |
| Turkey | 2015 | AFP contact | 2 | 0 | 1 | 0 | 0.7 | 96 | <1 yr | Jan 20, 2015 |
Abbreviations: AFP = acute flaccid paralysis; OPV = oral poliovirus vaccine; PID = primary immunodeficiency; SCID = severe combined immunodeficiency; XLA = X-linked agammaglobulinemia.
* Total years detected and cumulative totals for previously reported cVDPV outbreaks (Nigeria, Pakistan).
† Outbreaks list total cases clearly associated with cVDPVs. Some VDPV case isolates from outbreak periods might be listed as aVDPVs.
§ Total cases for VDPV-positive specimens from AFP cases and total VDPV-positive samples for environmental (sewage) samples.
¶ Percentage of divergence is estimated from the number of nucleotide differences in the VP1 region from the corresponding parental OPV strain.
** Coverage with 3 doses of oral poliovirus vaccine, based on 2014 data from the World Health Organization (WHO) Vaccine Preventable Diseases Monitoring System (2015 global summary) and WHO-UNICEF coverage estimates. National data might not reflect weaknesses at subnational levels. http://www.who.int/immunization/monitoring_surveillance/en/.
†† Duration of cVDPV circulation was estimated from extent of VP1 nucleotide divergence from the corresponding Sabin OPV strain; duration of immunodeficiency-associated VDPV replication was estimated from clinical record by assuming that exposure was from initial receipt of OPV; duration of ambiguous VDPV replication was estimated from sequence data.
§§ Three genetically linked isolates were classified as aVDPVs according to the VDPV guidelines (http://www.polioeradication.org/Portals/0/Document/Resources/VDPV_ReportingClassification.pdf), which require detection for >2 months.
 FIGURE 2. Number of circulating vaccine-derived poliovirus (cVDPV) cases detected, by serotype — worldwide, January 2000–May 2016*
FIGURE 2. Number of circulating vaccine-derived poliovirus (cVDPV) cases detected, by serotype — worldwide, January 2000–May 2016*
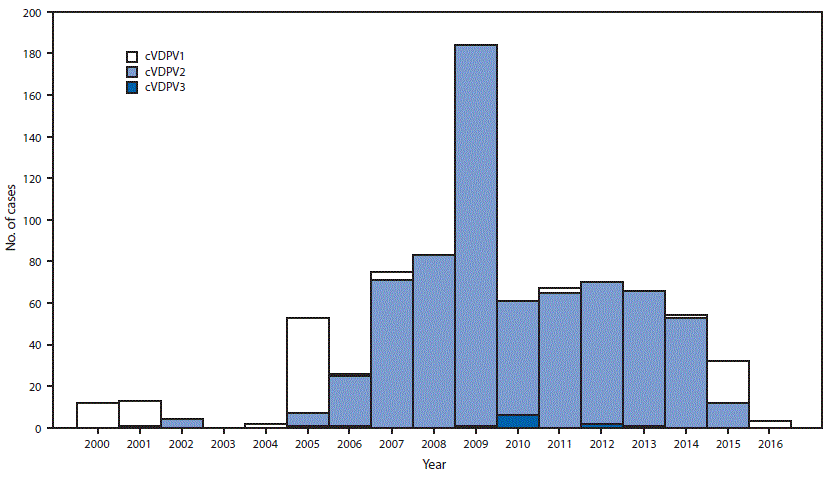
* Data through May 2016 as available by July 14, 2016.
Suggested citation for this article: Jorba J, Diop OM, Iber J, Sutter RW, Wassilak SG, Burns CC. Update on Vaccine-Derived Polioviruses — Worldwide, January 2015–May 2016. MMWR Morb Mortal Wkly Rep 2016;65:763–769. DOI: http://dx.doi.org/10.15585/mmwr.mm6530a3.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.
- Page last reviewed: August 17, 2017
- Page last updated: August 17, 2017
- Content source:


 ShareCompartir
ShareCompartir