VDSCP: Procedures
The goal of the CDC Vitamin D Standardization and Certification Program (VDSCP) is to improve the analytical accuracy and reliability of clinical vitamin D tests. Participants in this program obtain detailed information about the analytical biases and precision of their vitamin D tests. Tests that meet criteria for analytical accuracy and precision are certified.
The program has several key characteristics that are not available with other programs:
- It provides a panel of single-donor sera covering a wide range of vitamin D concentrations. This gives detailed information about measurement performance at multiple concentration levels and helps identify potential sources of measurement bias.
- It assesses measurement bias and imprecision by collecting data from replicate measurements.
- It monitors the consistency of participants’ vitamin D measurements by assessing measurement performance every quarter.
Assessments of analytical bias and precision are performed using well-recognized and established protocols.
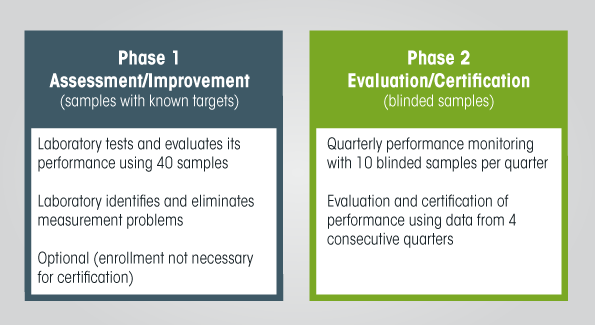
The typical procedure to achieve VDSCP standardization certification starts with an initial assessment of the participant’s testing performance. That is followed by enrollment in the actual certification process. Participants may, however, choose to do only one or the other.
In the initial assessment, participants submit a request to VDSCP for a set of samples to measure. The participants measure these samples and compare their results against those assigned by the CDC reference method to uncover analytical performance improvements that might be needed. VDSCP suggests following Clinical and Laboratory Standards Institute (CLSI) Protocol EP9-A, “Method comparison and bias estimation using patient samples,” for performing initial assessments. This document recommends a minimum of 40 samples. VDSCP can provide participants with various numbers of samples, up to 120 per set.
In the actual certification process, VDSCP sends 10 unknown samples to participants every quarter. Participants measure these samples and report the results to VDSCP. Each participant then receives a report about their measurement biases and imprecision every quarter.
VDSCP uses results from four consecutive quarters to assess measurement bias and imprecision, then issues a comprehensive report for each participant [PDF – 407 KB]. Participants meeting analytical performance criteria for bias and imprecision are certified.
What is the goal of VDSCP certification?
The goal of certification through VDSCP is to ensure that users of tests for total 25-hydroxyvitamin D produce accurate measurement results. This is achieved by standardizing tests at the manufacturer level. In this way, laboratories know that all new tests they receive are standardized. CDC’s program also offers certification to laboratories that use laboratory-developed tests or tests from manufacturers that were not certified.
What makes VDSCP unique?
CDC’s VDSCP is unique because it uses unmodified, high-quality serum samples for evaluating analytical bias and precision. This allows analytical performance assessment with sera similar to those used in patient care settings. The use of such sera is required because modified sera, such as those that are pooled or otherwise modified, can introduce so-called “matrix effects.” Such interferences are caused by any component of a sample other than the analyte itself. These matrix effects can lead to incorrect conclusions about the accuracy of a test.
What criteria are used to certify vitamin D?
As part of the certification process, the accuracy of tests for total 25-hydroxyvitamin D are compared with predefined analytical performance criteria. Tests meeting these criteria are considered standardized by VDSCP.
Analytical performance criteria for routine tests
used to measure total 25-hydroxyvitamin D
| Maximum Allowable Bias* | Maximum Allowable Imprecision (Coefficient of Variation) |
|---|---|
| ≤5.0% | <10.0% |
* Bias to the reference method.
CDC’s Vitamin D Reference Laboratory assigns reference values for vitamin D in the sera used in this program. The laboratory also provides information about the concentration of C3-epi-25-hydroxyvitamin D3 to participants.
Vitamin D Standardization Certification Program – Protocol [PDF – 661 KB]
- Page last reviewed: July 6, 2017
- Page last updated: July 6, 2017
- Content source:


 ShareCompartir
ShareCompartir