CRMLN: Cholesterol Reference Method Laboratory Network
The Cholesterol Reference Method Laboratory Network (CRMLN) is a network of highly specialized laboratories that work directly with manufacturers and laboratories to assess the analytical accuracy and precision of their tests. CRMLN provides reference measurement services to laboratories for measuring total cholesterol (TC), HDL-cholesterol (HDL-C), LDL-cholesterol (LDL-C), and total glycerides (TG). CRMLN laboratories have to meet stringent analytical performance criteria to ensure their measurements are sufficiently accurate and precise for evaluating these tests.
Network laboratories assess tests produced by manufacturers and laboratories for their analytical accuracy and precision. Tests and laboratories meeting established criteria for accuracy and precision are considered “CDC-certified,” and sufficiently accurate and precise for use in patient care. Lists of the clinical diagnostic products that have been certified through this process are updated regularly.
The Food and Drug Administration (FDA) has recognized the value of CDC’s Standardization Programs and work performed through CRMLN, and encourages manufacturers to be standardized to CDC.
What is the goal of certification through CRMLN?
The goal of certification through CRMLN is to ensure that users of tests for HDL-C, LDL-C, TC and TG produce sufficiently accurate and precise measurement results. These results are comparable to those used to derive current medical decision points for use in patient care. Therefore, health care providers can compare their patients’ results to current clinical decision points to consistently evaluate their health status, regardless of the test system used.
What are the reference methods used by CRMLN?
The CDC Lipids Reference Laboratory (LRL) and CRMLN Member Laboratories perform reference measurement procedures for total cholesterol, total glycerides, HDL-Cholesterol and LDL-cholesterol using traditional spectroscopic methods as well as new mass spectrometric methods. Current clinical decision points come from reference methods that used certain spectrophotometric measurements, an old form of measurement technology. New clinical decision points are being developed using newer mass spectrometry-based reference methods. Because old and new methods are currently in use, CDC’s CRMLN certification programs offers evaluations and certifications for both.
Why does standardization using CRMLN require single-donor sera?
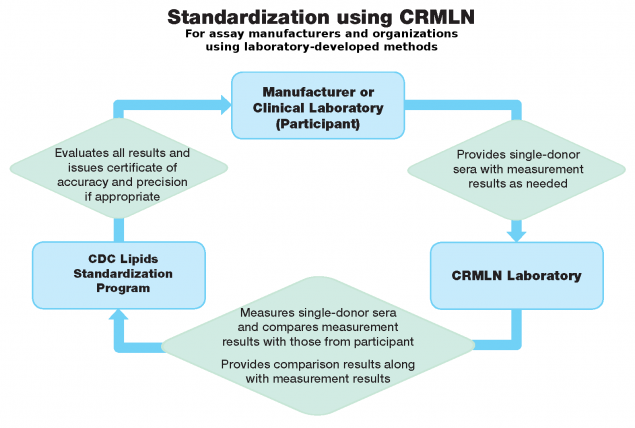
CDC’s manufacturer certification program is unique because it uses unmodified, high quality serum samples for evaluating the analytical accuracy and precision of commercial tests. This allows analytical performance assessment with sera similar to those used in patient care settings. The use of such sera is required because modified sera, such as those that are pooled or otherwise modified, can introduce so-called ‘matrix effects.’ These matrix effects can lead to incorrect conclusions about the accuracy of a test.
Is there a difference between certifying manufacturers and clinical laboratories?
There are two certification programs available for blood lipids: one is intended for manufacturers of tests (manufacturer certification); and the second for laboratory-developed tests, or laboratories with analytical systems not certified by the manufacturer (laboratory certification). However, any laboratory may participate in either laboratory certification program.
How are participants evaluated?
As part of the certification process, the accuracy and precision of tests for TC, HDL-C, LDL-C and TGs are compared to predefined analytical performance criteria. Comparison is performed following established protocols, such as those published by the Clinical and Laboratory Standards Institute.
Disclaimer
Use of trade names is for identification only and does not constitute endorsement by the CDC or the U.S. Department of Health and Human Services.
- Page last reviewed: July 6, 2017
- Page last updated: July 6, 2017
- Content source:


 ShareCompartir
ShareCompartir
