Domestic Intestinal Parasite Guidelines
On This Page
- Introduction
- Epidemiology
- History and evolution of the predeparture presumptive treatment for parasites in US-bound refugees
- Domestic guidelines for screening for parasitic infections
- No presumptive treatment
- Finding information sources on presumptive treatment received
- Management of asymptomatic refugees who received complete presumptive treatment for parasitic infection
- Screening and diagnostic tests for strongyloidiasis
- Screening and diagnostic tests for schistosomiasis
- Persistent eosinophilia in refugee populations
- Other parasitic infections commonly encountered in refugees
- Table 1: Recommended medication regimen for presumptive treatment of parasitic infections
- Table 2: Common parasites detected on stool examination
- Table 3: Causes of eosinophilia
- References
PDF version of these guidelines for printing [PDF – 14 pages]
Presumptive Treatment and Screening for Strongyloidiasis, Schistosomiasis and Infections Caused by Soil-Transmitted Helminths for Refugees
Introduction
It is estimated that more than one billion people are infected with soil-transmitted helminths (STH), 200 million with Schistosoma spp., and 100 million with Strongyloides.1 2 3 These parasites are some of the most common infections in refugees.4 5 Although frequently asymptomatic or sub-clinical, these infections may cause significant illness and even result in death. Parasites that infect humans represent a very complex and broad category of organisms. This section of the guidelines will address the parasitic infections most commonly encountered in refugees but will focus on soil-transmitted helminthic infections (Ascaris, Trichuris, hookworm), Strongyloides, and Schistosoma spp.
Epidemiology
Most refugees are at risk for parasitic infection. Prevalence data for parasitic infections in refugees in the United States are largely derived from stool and serologic surveys. These surveys have revealed high rates of certain infections in most refugee populations such as soil-transmitted helminth infections, Strongyloides and intestinal protozoal infections. In addition, they also show that there are some parasitic infections that are more frequently observed in certain populations such as Schistosoma spp. infections in sub-Saharan African (SSA) refugees. The overall prevalence of potentially pathogenic parasites among refugees resettled in North America has been reported to range from 8% to 86%.4 5 This broad range can be explained by differences in geographic origin, age, living conditions (including quality of drinking water, sanitation, and access to footwear), dietary habits, occupational history, education level, and previous countries of exposure/asylum in the populations studies.5 In addition, the variation in prevalence may be due to the type of sample tested (e.g., stool or serum) and methodologic differences among tests performed in different studies.6
Since the initiation of presumptive predeparture albendazole (1999), prevalence of soil-transmitted helminths detected among refugees following arrival in the United States have dramatically decreased.8 9 However, the regimen of a single dose of albendazole has little or no effect against other important parasitic infections. Of particular concern are two parasitic infections commonly encountered in refugee populations: Strongyloides stercoralis (nematode) and Schistosoma spp. (trematode). Strongyloides infection is found in many refugee populations but is particularly prevalent among Southeast Asian refugees. Schistosoma spp. infections are encountered predominantly in SSA refugees. The prevalence of these parasitic infections was initially under-recognized since most early studies utilized only routine stool testing, a poor technique for detection of these particular organisms.9 10 11 12 Both strongyloidiasis and schistosomiasis are considered chronic infections and both have been associated with morbidity and mortality years after migration.
As many as 100 million persons worldwide are estimated to be chronically infected with Strongyloides.5 Prevalence in serosurveys of refugee populations range from 11–69% infected. Unlike most parasitic infections which are unable to replicate in the human host, Strongyloides is able to replicate and auto-infect causing infection that may persist for decades. If the condition is not detected promptly after arrival, data indicate that the average time to diagnosis in the United States is more than 5 years after migration.11 In fact, one study found that 24% of Laotian refugees had continued strongyloidiasis an average of 12 years after migration.9 Strongyloides hyperinfection syndrome may occur many years after migration to a non-endemic setting, with case reports occurring >50 years after last known exposure.15 16 17 Hyperinfection syndrome, triggered when large numbers of parasites infiltrate internal organs, results in fatality rates exceeding 50%. The syndrome is generally induced when an individual is placed on corticosteroids although other immunosuppressive conditions such as cancer and transplant chemotherapeutic immunosuppression may also trigger hyperinfection syndrome. 15 17
More than 200 million people worldwide are estimated to be infected with schistosomiasis. The seroprevalence in SSA refugees ranges from 15-46%. Infection with schistosomiasis may persist in humans for more than 25 years and has been associated with many sequelae depending on the species, the parasite load and the host response. Schistosomiasis is associated with liver cirrhosis and resulting clinical complications (S. mansoni, S. japonicum), squamous cell carcinoma of the bladder (S. haematobium), and urinary tract obstruction and renal failure (S. haematobium). Potentially devastating clinical manifestations occasionally occur when the parasite egg enters the systemic circulation and travels to a normally sterile site within the body, causing severe inflammation. Eggs may travel to any part of the body, including the brain and spinal cord where the inflammatory response to the egg may cause paralysis or myelitis.
History and evolution of the predeparture presumptive treatment for parasites in US-bound refugees
In 1997, a CDC pilot project evaluated single dose albendazole presumptive treatment in U.S.-bound Barawan Somali refugees. This project demonstrated decreases in soil-transmitted parasites with presumptive treatment. In May 1999, CDC extended this recommendation to all refugees resettling from Africa and Asia. In 2008 the recommendation was extended to refugees from the Middle East. Currently, most refugees from these countries without a contraindication are receiving a single dose of albendazole prior to departure. A table of countries that are currently implementing these pre-departure presumptive treatments can be found here.
Data increasingly suggest that predeparture albendazole treatment has dramatically decreased the overall prevalence of soil-transmitted helminth infections in refugees. A recent evaluation of more than 26,000 African and Asian refugees demonstrated single dose albendazole resulted in an absolute reduction of the prevalence of any soil-transmitted helminth from 20.8% to 4.7% as measured by stool ova and parasite (O&P) examination. Albendazole-treated refugees were less likely than untreated refugees to have any nematode (prevalence ratio (PR), 0.19), Ascaris (PR 0.06), hookworm (PR 0.07), or Trichuris (PR, 0.27).8 These findings support previous data in African refugees resettling to the United States showing similar decrease in soil-transmitted helminths following implementation of predeparture albendazole treatment.7 However, despite this documented decrease in the overall prevalence of soil-transmitted helminth infections, a single dose of albendazole has very limited effect on infection with Strongyloides and no effect against Schistosoma spp.
Due to documented high rates of infection in refugee populations and increasing reports of serious clinical consequences, in 2005 CDC issued recommendations for predeparture treatment for strongyloidiasis (in all Middle Eastern, Asian, and African refugees) as well as for schistosomiasis (in SSA refugees only). These guidelines advise presumptive treatment for strongyloidiasis with 2 doses of ivermectin for refugees from non-Loa loa endemic countries. In addition, as of January 2010, presumptive predeparture treatment for schistosomiasis with praziquantel is recommended for SSA refugees who do not have contraindications (Table 1). However, institution of these recommendations was variable due to funding restrictions and logistical challenges. A table of countries that are currently implementing these pre-departure presumptive treatments can be found here.
Domestic guidelines for screening for parasitic infections
Predeparture presumptive treatment
Certain populations may not receive predeparture presumptive treatment. In addition, specific individuals within in each population may not be eligible for certain predeparture medications due to pregnancy, breastfeeding, young age, or other contraindications, such as known hypersensitivity or allergies and history of seizures or known neurocysticercosis. Thus, post-arrival guidelines are designed to be flexible and are contingent upon whether the predeparture presumptive treatment was received. Ideally, the clinician will have documentation of presumptive therapy received by each individual at the time of new arrival screening. However, it is recognized that currently this documentation is not consistently available. In cases when the documentation is not available it is reasonable to assume presumptive treatment has been received by the individual refugee if the refugee is from a population where the program is currently implemented and as long as they had no contraindications at the time of departure. CDC periodically monitors ongoing programs and has documented 75-100% compliance rates in populations that are currently treated. If a country does not appear on this table, then compliance rates in that country are uncertain, so the clinician should use their judgment regarding screening the refugee or providing presumptive treatment.
An algorithm has been developed based on current programming to assist the post-arrival clinician in the management of parasites in newly arriving refugees (Figure 1). Presently, the majority of resettling populations receive predeparture albendazole making stool testing or treatment for soil-transmitted helminths (STH) unnecessary for most refugees. In addition, the majority of SSA populations have been presumptively treated for schistosomiasis with praziquantel; therefore, domestic testing and treatment for schistosomiasis are also not necessary for most refugees from SSA. Refugees arriving from the Middle East, Asia, and SSA countries that are not endemic for L. loa, are at high risk for strongyloides infection. If they have not been presumptively treated overseas with ivermectin, they should receive presumptive treatment with ivermectin after arrival or undergo testing followed by treatment, if positive. A table of countries that are currently implementing these pre-departure presumptive treatments can be found here. It is anticipated that the implementation of predeparture presumptive treatment for strongyloidiasis may be expanded to other US-bound refugee populations in the future so clinicians should continue to check the CDC website for updated information.
Refugees from L. loa endemic SSA countries should not receive presumptive ivermectin due to the possible risk of encephalopathy associated with a high load of dying microfilaria. After arrival in the United States, they should undergo testing for strongyloidiasis. Those found to be infected may be treated with a longer course of high-dose albendazole (400 mg orally twice a day for 7 days). Alternatively, they may be screened for L. loa with a daytime blood smear and if not infected with L. loa, they can receive presumptive treatment with ivermectin.
The following sections recommend approaches for evaluating refugees during the post-arrival domestic medical visit based on whether the refugee had documented or presumed predeparture treatment. For the purposes of post-arrival management of parasites, refugees may be divided into three groups: no predeparture presumptive treatment, incomplete presumptive treatment, and complete presumptive treatment.
No presumptive treatment
Refugees in this category did not receive any presumptive treatment for parasites prior to departure for the United States. This group includes persons from populations not included in the table of presumptive treatment programs and those excluded due to contraindications to presumptive treatment with albendazole, praziquantel, and ivermectin. An algorithm has been developed to assist clinicians in managing patients who have received no predeparture therapy either because they were from a population not included in current programming, or if they had a contraindication at the time of departure (Figure 1).
- Albendazole contraindications
- Children < 1 year of age, pregnant women, refugees with known neurocysticercosis, evidence of cysticercosis (e.g., subcutaneous nodules), or with a history of unexplained seizures
- Praziquantel contraindications
- Children < 4 years of age or measuring < 94 cm, refugees with known neurocysticercosis, evidence of cysticercosis (e.g., subcutaneous nodules), or with a history of unexplained seizures.
- Ivermectin contraindications
- Children < 15 kg or measuring < 90 cm, pregnant women in any trimester or breastfeeding women within the first week after birth.
- Refugee is departing from or has lived in a Loa loa endemic area.
Incomplete presumptive treatment
Refugees in this category did not receive all of the recommended overseas presumptive treatment for parasites prior to departure. An algorithm has been developed to assist clinicians on post-arrival management for those who received incomplete therapy (Figure 2).
- Most refugee populations are receiving single dose albendazole and it is reasonable to assume they have received albendazole if they are from populations included in the presumptive treatment programs and do not have a contraindication to albendazole.
- Most SSA refugees, are receiving predeparture praziquantel treatment for schistosomiasis if they are from populations included in the presumptive treatment programs and do not have a contraindication to praziquantel.
Complete presumptive treatment
Refugees in this category received all recommended treatments for overseas presumptive treatment for parasites prior to departure (Figure 3). A table of countries that are currently implementing these pre-departure presumptive treatments can be found here.
Finding information sources on presumptive treatment received
There have been substantial challenges in providing the records of presumptive parasite treatment for individual refugees to US clinicians at the time of their new arrival medical examination. Therefore, information and individual refugee presumptive treatment records are made available in multiple formats.
- IOM Bag: Records of presumptive predeparture treatment received by the refugee are usually available in the IOM bag, also known as the “blue and white bag,” carried by the refugee. The refugee should be directed to bring the IOM bag to the clinic at the time of appointment. The provider may also check with the voluntary resettlement agency coordinating the refugee’s care as they may have a copy of these records.
- Electronic Disease Notification System (EDN): Records will be made available to Refugee Health Coordinators and some clinics through EDN. Clinicians may request this information from their respective state refugee health program.
- CDC Website: In addition, refugee populations receiving presumptive treatment will be listed on CDC’s website. A table of countries that are currently implementing these pre-departure presumptive treatments can be found here.
Summary of management of parasitic infections by population in asymptomatic refugees
Management of asymptomatic refugees who received no predeparture treatment
- A refugee who received no overseas predeparture antiparasitic treatment should receive either post-arrival presumptive treatment or post-arrival screening.
- If presumptive treatment is selected, follow the recommendations in Table 1. If screening is selected, follow the algorithm in Figure 1.
Management of asymptomatic refugees who received incomplete presumptive treatment for parasitic infection
- A refugee who received incomplete presumptive treatment overseas should receive either post-arrival presumptive treatment or post-arrival screening (Figure 2).
- If presumptive treatment is selected, follow the recommendations in Table 1.
Most refugees resettling in the United States who fall into this category have received albendazole and do not require routine stool O&P screening or presumptive treatment with albendazole. Although most refugees currently receive albendazole therapy, many are not receiving treatment for strongyloidiasis or schistosomiasis even though such treatment was indicated. Depending on which treatment was not received, the clinician will need to select the appropriate presumptive treatment or screen and treat strategy. Presumptive treatment for strongyloidiasis for refugees from non-Loa loa endemic countries is two doses of ivermectin given on two consecutive days (Table 1). For those from Loa loa endemic areas, a screen and treat approach is usually more appropriate, although presumptive high-dose, long-course albendazole with or without screening is an acceptable alternative for presumptive treatment of Loa loa (Table 1). For SSA refugees who have not received predeparture therapy for schistosomiasis, post-arrival presumptive treatment with praziquantel (Table 1) or screening and treatment is indicated. Serologic testing is the most sensitive test for screening. Details on diagnostic tests available for Strongyloides and schistosomiasis in symptomatic patients are discussed below.
Management of asymptomatic refugees who received complete presumptive treatment for parasitic infection
- There is no need to screen asymptomatic refugees for parasitic infection if they received the complete treatment package (Figure 3).
However, it is common for arriving refugees to have a screening eosinophil count as part of their routine complete blood count (CBC) with differential. If elevated this may indicate infection and follow-up is recommended in Figure 3.
Screening and diagnostic tests for strongyloidiasis
Screening for strongyloidiasis involves serologic testing or stool culture. Stool ova and parasite examination may be used, but testing of three samples results in less than 60% sensitivity and up to 7 samples may be necessary to increase sensitivity to more than 90%. Serologic testing is the most widely available testing modality and positive results are sufficient to confirm the diagnosis. However, a combination of more than one testing technique may increase sensitivity and specificity (e.g., serology and stool ova and parasite testing). For SSA refugees who have lived in areas endemic for L. loa, screening for L. loa using a quantitative daytime blood smear or filariasis/Loa loa serology is necessary in order to determine the safety of ivermectin therapy. Refugees who are negative may be treated with ivermectin; those who are positive may be treated with high dose albendazole. Testing for Loa loa is not necessary if treating persons with strongyloidiasis with high-dose albendazole. Further information on loiasis is available on the CDC Division of Parasitic Diseases Website.
Screening and diagnostic tests for schistosomiasis
Serologic testing is the most sensitive test for screening for schistosomiasis. Stool ova and parasite testing may detect infection, although, similar to testing for strongyloidiasis, sensitivity can be quite low. Diagnosis of schistosomiasis can be made using stool, urine studies, or serologies. Refugees at risk for S. hematobium should have urine checked for ova. Schistosoma hematobium is endemic to much of Africa, parts of Arabia, the Middle East, Khuzestan Province in Iran, Madagascar, and Mauritius. Those who are from areas endemic for the other schistosome species should have stool checked for ova to detect other species such as S. mansoni and S. japonicum. Standard stool studies are not as sensitive for schistosomiasis as for soil-transmitted helminths. When performing stool testing for schistosomiasis it is important to collect three specimens on three separate days. Although the Kato Katz method(staining a stool sample and examining under a microscope) is preferred, formalin preserved stool with concentration may also detect schistosome ova. Urine should also be collected three times on three separate days. Hematuria can be used as an indicator of infection in persons from endemic areas in SSA. Serologic testing is useful for diagnosis in populations that have never received praziquantel. Combining serology and stool testing may increase sensitivity and specificity in both screening and in diagnosis of disease. In addition, other findings on routine screening tests may suggest infection such as eosinophilia (e.g., strongyloidiasis, schistosomiasis) or hematuria (e.g., schistosomiasis), although these are non-specific findings.
Further information on individual parasites may be obtained from www.cdc.gov/parasites and assistance with diagnosis or management of parasitic diseases may be obtained through the CDC’s Parasitic Diseases Branch Public Inquiries Line at parasites@cdc.gov or 404-718-4745.
Persistent eosinophilia in refugee populations
Figure 3 describes management of baseline eosinophilia in an asymptomatic refugee who is tested upon resettlement in the United States. Ideally, the eosinophil count would be checked in refugees only 3-6 months after receipt of the complete presumptive treatment. In practice, most refugees receive a baseline eosinophil count as part of a CBC with differential done as part of the new arrival medical screening that occurs shortly after presumptive predeparture treatment and resettlement in the United States. If the eosinophil count is normal, repeat testing for eosinophilia in an asymptomatic person is not indicated. If the absolute eosinophil count is ≥ 400 cells/mL clinical decision making should be based on the history of presumptive treatment.
- Refugees who received the complete presumptive treatment package should have their eosinophil count repeated in 3-6 months, as it can take this long for eosinophilia to resolve after appropriate treatment. No other testing is indicated in an asymptomatic refugee.
- If the refugee has not received the complete presumptive treatment package it is reasonable to provide the missing components of the complete treatment package (Table 1) and recheck eosinophil counts in 3-6 months after receipt of treatment prior to embarking on a diagnostic work-up. The most common etiologies of eosinophilia in asymptomatic refugee populations are soil-transmitted helminths, Strongyloides, and Schistosoma infections.
- If the refugee has not received the complete presumptive treatment package and the clinician opts for screening for parasitic infections in lieu of presumptive treatment, the work-up suggested in Figure 1 may be followed. The most common etiologies of eosinophilia in asymptomatic refugee populations are soil-transmitted helminths, Strongyloides, and Schistosoma infections.
If the eosinophilia persists 6 months after treatment, an evaluation of eosinophilia that includes other parasites (other than soil-transmitted helminths, Strongyloides, and Schistosoma infections) as well as non-parasitic causes should be considered.18
Other parasitic infections commonly encountered in refugees
There are many parasitic infections encountered in refugees which may be detected as a result of solicited symptoms such as abdominal pain (e.g., Hymenolepis nana) or seizures (cystercercosis), physical examination (e.g., filariasis, onchocerciasis), laboratory abnormalities such as persistent eosinophilia (e.g., liver or lung flukes) or as incidental findings on stool examination (e.g., protozoal infections). Further information on these parasitic infections and assistance with diagnosis and treatment may be provided by the Centers for Disease Control and Prevention’s Division of Parasitic Diseases.
Table 1. Recommended medication regimen for presumptive treatment of parasitic infections
Adults
| Refugee Population | Regimens by Pathogen | ||
|---|---|---|---|
| Soil-transmitted helminths: Albendazole | Strongyloidiasis: Ivermectin or high-dose albendazole | Schistosomiasis: Praziquantel1 | |
| Asia, Middle East, and North Africa, Latin America and Caribbean | 400 mg orally for 1 day | Ivermectin, 200 μg/kg/day orally once a day for 2 days | Not recommended |
| Sub-Saharan Africa, non-Loa loa-endemic area | 400 mg orally for 1 day | Ivermectin, 200 μg/kg/day once a day for 2 days | Praziquantell, 40 mg/kg (may be divided and given in two doses for better tolerance). |
| Sub-Saharan Africa, Loa loa– endemic area | 400 mg orally for 1 day | Only use ivermectin (200 μg/kg/day once a day for 2 days) if Loa loa infection has been ruled out. May use high dose albendazole (400 mg twice a day for 7 days)) if Loa loa infection cannot be ruled out. For more information see screening and diagnostic tests for strongyloidiasis below. | Praziquantel, 40 mg/kg (may be divided and given in two doses for better tolerance). |
Pregnant women
| Refugee Population | Regimens by Pathogen | ||
|---|---|---|---|
| Soil-transmitted helminths: Albendazole | Strongyloidiasis: Ivermectin or high-dose albendazole | Schistosomiasis: Praziquantel1 | |
| Asia, the Middle East/North Africa, Latin America and Caribbean | Not recommended | Not recommended | Not recommended |
| Sub-Saharan Africa | Not recommended | Not recommended | Praziquantel, 40 mg/kg (may be divided and given in two doses for better tolerance). |
Children
| Refugee Population | Regimens by Pathogen | ||
|---|---|---|---|
| Soil-transmitted helminths: Albendazole | Strongyloidiasis: Ivermectin or high-dose albendazole | Schistosomiasis: Praziquantel1 | |
| Asia, the Middle East/North Africa, Latin America and Caribbean | 12-23 months of age: 200 mg orally for 1 day. Presumptive therapy is not recommended for any infant less than 12 months of age. | Ivermectin, 200 μg/kg/day orally once a day for 2 days Should not be used presumptively if ≤15 kg or from Loa loa-endemic country. |
Not recommended |
| Sub-Saharan Africa | 12-23 months of age: 200 mg orally for 1 day. Presumptive therapy is not recommended for any infant less than 12 months of age. | Ivermectin, 200 μg/kg/day orally once a day for 2 days Should not be used presumptively if ≤15 kg or from Loa loa-endemic country. |
Children under ≤4 years of age should not receive presumptive treatment with praziquantel. Only for children from sub-Saharan Africa |
Although WHO states ivermectin and albendazole may be given concurrently, it is recommended that ivermectin be taken on an empty stomach and albendazole with fatty foods.
Praziquantel, if not co-administered, should be administered at least one day prior to either ivermectin or albendazole. Praziquantel should be taken with liquids during a meal.
All sub-Saharan African countries are considered endemic for schistosomiasis except Lesotho.
Table 2. Common parasites detected on stool examination
| Pathogenic | Controversial | nonpathogenic | ||||
|---|---|---|---|---|---|---|
| Nematodes | Trematodes | Cestodes | Protozoa | Protozoa | Other | Protozoa |
| Ascaris lumbricoides Hookworm (Necator americanus & Ancylostoma duodenale) Trichuris trichiura Strongyloides stercoralis |
Schistosoma (S. mansoni, S. haematobium, S. japonicum) Other flukes (Ophisthorchis spp.) Fasciola, Paragonimus westermani) |
Taenia solium Taenia. saginatum Hymenolepis nana |
Entamoeba histolytica* Giardia intestinalis (also known as G. lamblia or G. duodenalis) |
Dientamoeba fragilis (diarrhea) Entamoeba polecki (diarrhea) |
Blastocystis hominis (diarrhea) | Entamoeba dispar* Entamoeba moshkowskii* Entamoeba coli Entamoeba hartmanii Endolimax nana Iodamoeba butschlii Chilomastix mesnili |
*The cyst of E. histolytica, E. dispar and E. moshkowskii are morphologically identical by stool microscopy (morphologically). When cysts are detected, stool antigen testing is recommended distinguish the potentially pathogenic E. histolytica from the more common, non-pathogenic species.
Table 3. Causes of eosinophilia
| Parasites that cause eosinophilia commonly found in stool examination | Other parasitic infections associated with eosinophilia | Parasites commonly found in the stool NOT typically associated with eosinophilia | Non-parasitic causes of eosinophilia |
|---|---|---|---|
| Ascaris lumbricoides Hookworm (Ancylostoma spp, Necator spp) Trichuris trichiura Strongyloides stercoralis* Tapeworm (Taenia solium and T. saginatum) Schistosoma (most commonly S. mansoni*, S. haematobium*, S. japonicum)* Other flukes (Paragonimus spp.*, Ophisthorchis spp.*, Fasciola spp.*) |
Angiostrongylus Anasakis Capillaria spp. (Cysticercosis) Echinococcus spp. Filariasis (Wuchereria bancrofti, Brugia spp, Mansonella spp, Onchocerca volvulus, Dracunculus medinensis. Loa loa) Schistosoma (most commonly S. mansoni*, S. haematobium*, S. japonicum*) |
Entamoeba spp. (histolytica/, dispar, other Entamoeba spp.) Cryptosporidium spp. Giardia intestinalis (also known as G. lamblia or G. duodenalis) |
Asthma Atopy Drug allergy Eosinophilic leukemia Hodgkin’s lymphoma Hyper- eosinophilic syndrome Pemphigoid Pemphigus Polyarteritis nodosa |
*Particularly common causes of eosinophilia which may be found in stool but special testing and/or multiple samples are frequently needed.
Figure 1. Management of asymptomatic refugees for parasitic infection if they received no pre-departure treatment as of March 1, 2013
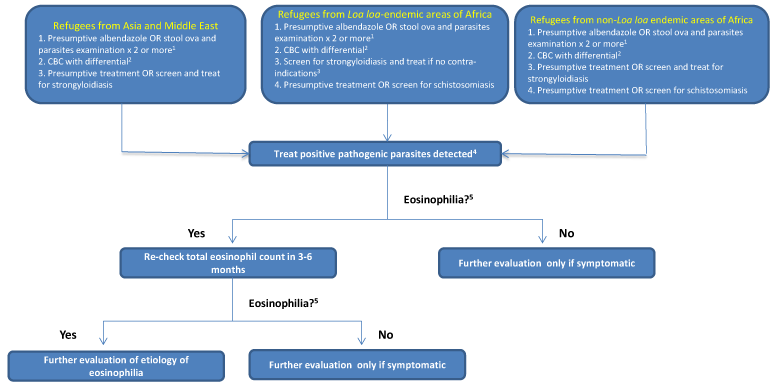
- 1Sensitivity varies according to parasite (e.g., very sensitive for Ascaris but may need 7 specimens to reliably exclude Strongyloides) and minimum of two specimens are suggested.
- 2CBC: Complete blood count and differential (not recommended as screening test for parasitic infection but routinely obtained on screening for newly arrived refugees)
- 3See text for discussion of screening for Loa loa
- 4See DPDx Laboratory Identification of Parasites of Public Health Concern. Diagnostic and management assistance may be obtained by contacting Division of Parasitic Diseases at CDC.
- 5Eosinophilia = a eosinophil count of >400 per microliter (μL)
Figure 2. Management of parasitic infections for refugee population currently receiving parasitic predeparture presumptive albendazole but incomplete predeparture therapy as of March 1, 20131
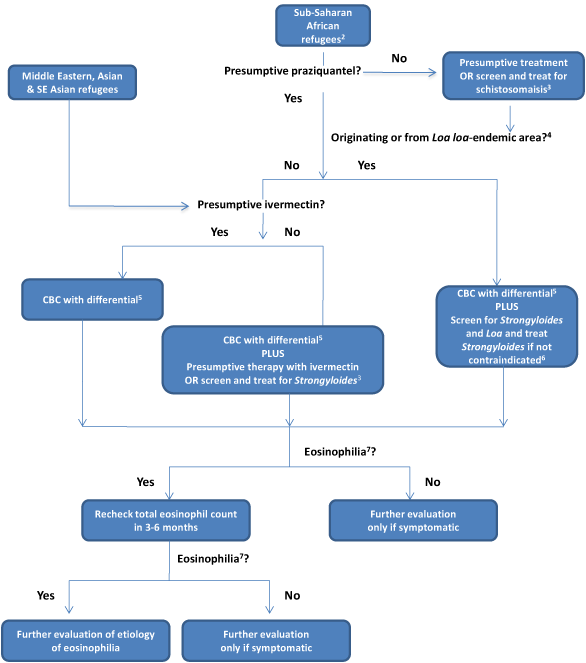
- 1Most refugees without a contraindication are currently receiving pre-departure albendazole. Visit the CDC’s current immunization schedule for US-bound refugees for current status of pre-departure presumptive treatment.
- 2Sub-Saharan African refugees without a contraindication are currently receiving pre-departure praziquantel for schistosomiasis. (Consider serologic screening for those with a contraindication at departure; see Figure 2.)
- 3See text for screening strategies
- 4See list 1 of overseas guidelines and map of Loa loa-endemic countries.
- 5CBC: Complete blood count and differential (not recommended as screening test for parasitic infection but routinely obtained for new arrival screening)
- 6See text for discussion of screening for Loa loa
- 7Eosinophilia = an eosinophil count of >400 μL.
Figure 3. Management of parasitic infection for asymptomatic refugees who received complete pre-departure therapy as of March 1, 2013
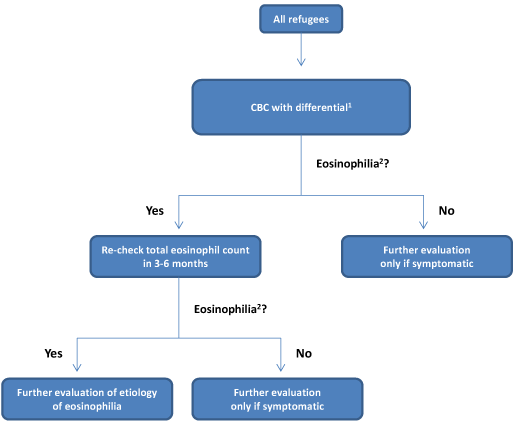
- 1 Complete blood count and differential (not recommended as screening test for parasitic infection but routinely obtained on screening for newly arrived immigrants and refugees)
- 2Eosinophilia = a eosinophil count of >400 μL
References
- de Silva NR, Booker S, Hotez PJ, et al. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol 2003;19:547-51.
- Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: Ascaris, trichuriasis, and hookworm. Lancet 2006;367:1521-32.
- Hotez PJ, Molyneux DH, Fenwick A, et al. Control of neglected tropical diseases. N Engl J Med 2007;357:1018-27.
- Peterson MH, Konczyk MR, Ambrosino, K, et al. Parasitic screening of a refugee population in Illinois. Diagnostic Microbiology and Infectious Disease 2001;40(1):75-76.
- Mody RK. Intestinal parasites. In: Walker PF, Barnett ED, eds. Immigrant Medicine. Vol 1. 1st ed. Philadelphia: Elsevier; 2007.
- Lurio J Verson H, Karp S. Intestinal Parasites in Cambodians: comparison of diagnostic methods used in screening refugees with implications for treatment of populations with high rates of infestation. J Am Board Fam Pract. 1991;4:71-8.
- Geltman PL, Cochran J, Hedgecock C. Intestinal parasites among African refugees resettled in Massachusetts and the impact of an overseas predeparture treatment program. Am J Trop Med Hyg. 2003;69:657-662.
- Swanson SJ, Phares CR, Mamo B, et al. Albendazole treatment and enteric parasites in United States-bound refugees. N Engl J Med 2012;366:1498-507.
- Caruana SR, Kelly HA, Ngeow JY, et al. Undiagnosed and potentially lethal parasite infections among immigrants and refugees in Australia. J Travel Med. 2006;13:233-239.
- Van Doorn HR, Koelewijn R, Hofwegen H, et al. Use of enzyme-linked immunosorbent assay and dipstick assay for detection of Strongyloides stercoralis infection in humans. J Clin Microbiol. 2007;45:438-442.
- Boulware DR, Stauffer WM, Hendel-Paterson BR, Rocha J, Chee-Seong Seet R, Andrea P. Summer AP, et al. Maltreatment of Strongyloides infection: case series and worldwide physician–in-training survey. Am J Med. 2007;120(60):545;e1-8.
- de Silva S, Saykao P, Kelly H, et al. Chronic Strongyloides stercoralis infection in Laotian immigrants and refugees 7-20 years after resettlement in Australia. Epidemiol Infect 2002:128(3):439-44.
- Posey DL, Blackburn BG, Weinberg M, Flagg EW, Ortega L, Wilson M, et al. High prevalence and presumptive treatment of schistosomiasis and Strongyloides among African refugees. Clin Infect Dis. 2007;45(10):1210-5.
- de Silva S, Saykao P, Kelly H, et al. Chronic Strongyloides stercoralis infection in Laotian immigrants and refugees 7-20 years after resettlement in Australia. Epidemiol Infect 2002:128(3):439-44.
- Lim S, Katz K, Krajden S, et al. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. CMAJ 2004:171:479-84.
- Gill GV, Beeching NJ, Khoo S, et al. A British Second World War veteran with disseminated strongyloidiasis. Trans Roy Soc Trop Med Hyg 2004: 98:382-6.
- Newberry AM, Williams DN, Stauffer WM, et al. Strongyloides hyperinfection presenting as acute respiratory failure and gram-negative sepsis. Chest 2005;128(5):3681-4.
- Seybolt LM, Christiansen D, Barnett ED. Diagnostic evaluation of newly arrived asymptomatic refugees with eosinophilia. Clin Infect Dis 2006:42(3):363-7.
- Page last reviewed: March 29, 2012
- Page last updated: June 17, 2013
- Content source:



 ShareCompartir
ShareCompartir