Screening for Hepatitis During the Domestic Medical Examination
PDF version of these guidelines for printing [PDF – 17 pages]
Introduction
Hepatitis is defined as inflammation of the liver. Hepatitis may result from any of a number of infectious and noninfectious causes. Many viral infections can cause liver inflammation, but the term viral hepatitis is usually reserved for infections with one of the five hepatotropic viruses (viruses known to target the human liver); these five agents are hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), and hepatitis E virus (HEV).
Infections caused by each virus vary in their epidemiologic features and natural history, including incubation period, routes of transmission, geographic and demographic distribution, patterns of clinical disease, and propensity for becoming chronic. Similarly, strategies for prevention and approaches to clinical management also vary by type of viral hepatitis.
Each of the five types of viral hepatitis may cause illness during acute infection (2 weeks to 6 months following exposure); while acute viral hepatitis can be severe or fatal, it is often asymptomatic. Acute infections caused by HBV and HCV commonly become chronic, persisting for decades and often silently causing liver damage. Chronic HBV and HCV infections result in a high disease burden and are leading causes of cirrhosis and hepatocellular carcinoma in the United States and globally. Since chronic HBV and HCV are frequently asymptomatic, it is imperative to consider these infections during the new arrival refugee screening examination. Hepatitis D virus (HDV) infection can propagate only in the presence of hepatitis B virus. Chronic HBV/HDV co-infection is more likely to progress to advanced liver disease than HBV monoinfection, and it increases the risk for development of hepatocellular carcinoma. HAV and HEV cause predominantly acute hepatitis and are the leading cause of symptomatic viral hepatitis infections globally. Although HAV has never been reported to cause chronic infection, there are rare reports of chronic HEV infection, particularly in immunosuppressed persons.
Chronic Viral Hepatitis
Hepatitis B Virus (HBV)
Background
Chronic hepatitis B virus (HBV) infection, defined as hepatitis B surface antigen (HBsAg) positivity for at least 6 months, is a major cause of preventable morbidity and mortality worldwide. Globally, more than 240 million people are chronically infected, leading to more than 786,000 deaths per year. The overwhelming majority of these deaths occur in resource-limited countries. Approximately 45% of the world’s population lives in areas of high endemicity, where the prevalence of chronic HBV infection is ≥8%, and the lifetime risk of acquiring HBV infection is > 60%. Another 43% live in areas of intermediate endemicity, where the HBsAg prevalence is 2%-7% and the lifetime risk of infection is 20%-60%. The remaining 12% live in areas of low endemicity, where the HBsAg prevalence is <2% and the lifetime risk of infection is <20%. Most refugees arriving in the United States come from countries of intermediate and high HBV endemnicity (Figure 1).
Persons with chronic HBV infection are at risk for developing HBV-related chronic liver disease, including cirrhosis and hepatocellular carcinoma, and extrahepatic manifestations, such as glomerulonephritis. Approximately 15%-25% of persons with chronic HBV infection will die prematurely from HBV-related cirrhosis or hepatocellular carcinoma.
Although usually asymptomatic, persons (including refugees) with chronic HBV infection are a potential source for transmitting infection to others. Common modes of HBV transmission include perinatal, sexual contact, and needle sharing. Transmission may also occur within households. More details on prevention and screening of HBV are available in the CDC publication Recommendations for Identification and Public Health Management of Persons with Chronic Hepatitis B Virus Infection. Identification of HBV-infected persons during the new arrival medical examination is an opportunity to decrease morbidity and mortality in resettling refugees, and to prevent transmission to family members and close contacts in the community.
Epidemiology of HBV infection in refugees
HBV infection prevalence and transmission patterns vary markedly among different countries and refugee populations. In general, prevalence rates of chronic HBV infection in migrant populations reflect the rates in the region of birth (Figure 1). Highly endemic regions (HBsAg ≥8%) include most of Asia, areas of Latin America, particularly the Amazon Basin, the South Pacific Islands, and sub-Saharan Africa. Populations with the highest documented chronic HBV infection rates have originated in East Asia, Southeast Asia, and the Pacific (12%-18% prevalence in most populations studied).
In highly endemic regions, new infections occur predominantly among infants and young children and result from perinatal or household transmission. The sequelae of chronic HBV infection are largely related to the age at time of infection. Acquisition during infancy or childhood is associated with a higher likelihood of leading to chronic infection and hepatocellular carcinoma; 25% of persons who become chronically infected as infants and 15% of persons who become chronically infected at an older age die of cirrhosis, hepatocellular carcinoma, or end-stage liver disease.
Testing for HBV infection
The serologic patterns of HBV infection are complex, and a complete discussion is beyond the scope of this document. Briefly, antigens and antibodies associated with HBV infection include HBV surface antigen (HBsAg) and antibody to HBsAg (anti-HBs), HBV core antigen (HBcAg) and antibody to HBcAg (anti-HBc), and HBV e-antigen (HBeAg) and antibody to HBeAg (anti-HBe). At least one serologic marker is present during each phase of HBV infection. The serologic markers most widely used in diagnosis of acute, resolving, and chronic HBV infection are HBsAg, total and IgM anti-HBc and anti-HBs. Table 1 outlines the typical interpretation of HBV serology. For more information on HBV and interpretation of hepatitis B serologic markers, please refer to the CDC website for Hepatitis B FAQs for Health Professionals.
Table 1. Hepatitis B serologic marker interpretation
| Serologic Marker | Interpretation | ||||
|---|---|---|---|---|---|
| HBsAg (Hepatitis B surface antigen) |
Total anti-HBc (Antibody to hepatitis B core antigen) |
IgM anti-HBc (Immunoglobulin M to anti-HBc) |
Anti-HBs (Antibody to HBsAg) |
||
| Test results | – | – | – | – | Never infected and susceptible to infection |
| + | + | – | – | Chronic infection | |
| – | + | – | + | Recovered from past infection and immune | |
| + | + | + | – | Acute infection | |
| – | + | – | + | Immune1 by natural infection | |
| – | – | – | + | Immune1 by hepatitis B vaccination | |
| – | + | – | – | Immune by natural infection or possible false positive | |
1Refugees who are partially vaccinated should complete the series
Recommendations for screening for chronic HBV in Refugees
During the medical examination for newly arrived refugees, screening for chronic HBV infection, including serologic assays for HBsAg, anti-HBc and anti-HBs, should be routinely performed on all refugees who are from or have lived in countries with intermediate (≥ 2%-7%) or high (≥8%) prevalence of chronic HBV infection (Figure 1, Figure 2). The only exception would occur if a negative hepatitis B surface antigen (HBsAg) test result is documented on their overseas medical forms. Most refugee groups resettling to the United States originate from or have lived in countries with intermediate or high hepatitis B endemicity. Vaccination should be offered to those who are not hepatitis B infected (negative HBsAg), who are nonimmune (negative anti-HBc and anti-HBs), and who are considered at high risk for infection (Figure 2). When interpreting serologies, if a partial vaccine series is documented (the refugee has received an incomplete series of hepatitis B vaccine, which is common in refugees), isolated anti-HBs positivity does not indicate sustained immunity, and the vaccine series should be completed based on the Advisory Committee on Immunization Practices (ACIP)-recommended schedule.
Adults
- All adults ≥18 years of age who were born or lived in countries where the rate of chronic hepatitis B virus infection is ≥2% (intermediate or highly endemic countries), and who have no documentation of a negative HBsAg on the overseas medical forms, should be tested for:
- hepatitis B surface antigen (HBsAg)1 regardless of vaccination history
- hepatitis B core antigen (anti-HBc),
- and hepatitis B surface antibody (anti-HBs)2
- All adults ≥18 years of age who were born or lived in countries where the rate of chronic hepatitis B virus infection is <2% should be tested as above only if they belong to a high-risk group, including:
- Men who have sex with men
- Persons with a history of injection drug use
- HIV-infected persons
- Household contacts of persons with chronic HBV infection
- Persons from low-prevalence countries who are part of subpopulations that have known prevalence rates of 2% or greater (i.e., indigenous populations)
- Persons on hemodialysis therapy
- Persons who have previously received whole blood or blood product transfusions
- Persons with elevated ALT/AST of unknown etiology
- Persons with medical conditions that require immunosuppressive therapy
- Pregnant women
Screening pregnant women for HBsAg is particularly important. Without prophylaxis starting at birth, 90% of infants born to an HBeAg-positive woman and 30% of those born to an HBeAg negative woman will develop chronic infection. Of these, 25% will die from cirrhosis, liver failure, or hepatocellular carcinoma. Early identification of maternal infection can enable pre- and postnatal counseling and ensure post exposure management to prevent transmission to the infant.
1Testing for HBsAg should not be performed within 30 days of receipt of hepatitis B vaccine 1, 2.
2If a partial series of hepatitis B vaccine is recorded, testing for anti-HBs is not necessary.
Children
- All refugee children <18 years of age who were born in countries where the rate of chronic HBV infection is ≥2% (intermediate or high-endemicity countries) should be tested for HBsAg, regardless of vaccination history (Figure 3).2 The only exception would occur if a negative hepatitis B surface antigen (HBsAG) test result is documented on their overseas medical forms. Checking anti-HBs and total anti-HBc may be reasonable in older children in populations with high prevalence rates (>8%) or who are at risk of infection.
- Although screening for HBsAg is recommended for all refugee children from countries with intermediate or high (≥2%) prevalence of HBV infection, some experts do not routinely check anti-HBc or anti-HBs in children from countries with HBV prevalence of <8%, to avoid false-positive results and because of concerns that routine testing in this group is not cost-effective. However, factors that increase the pre-test probability for past or current HBV infection should prompt testing for anti-HBc and anti-HBs. These factors include:
- Children with an immediate family member, particularly the mother, who is chronically infected with HBV.
- Children from a low-prevalence country who are members of a subpopulation with prevalence of ≥2% (e.g., some indigenous populations)
- HIV-infected children
- Children who received whole blood products or blood components prior to migration.
- Children who are injection drug users or who have multiple sex partners or have a history of sexual exploitation.
- All children ≤18 years old who are HBsAg negative should receive the complete hepatitis B vaccination series according to ACIP guidelines. The vaccine series can be started while waiting for HBV screening test results.
- Any refugee child who had a potential exposure to HBV within 60 days of the new arrival examination should have repeat testing 3-6 months after arrival.
All HBsAg-positive persons should receive appropriate counseling and be evaluated for treatment. Persons living in households or other crowded living conditions with a chronically infected person should be tested for hepatitis B and, if susceptible, receive the 3-dose hepatitis B vaccine series.
In addition, any refugee who has had a potential exposure to HBV within 60 days of the new arrival examination should have repeat testing 3-6 months after arrival. Such refugees should be advised to seek medical care if hepatitis-related symptoms (e.g., jaundice, nausea/vomiting, right upper quadrant pain) occur during this period.
Vaccination records
An increasing number of countries are including hepatitis B in their routine vaccination schedules. Initiation of the vaccine series prior to departure for U.S.-bound refugees is also increasing, particularly in populations originating in South and Southeast Asia. All refugees from intermediate- (2%-7%) or high (≥8%)-endemicity countries should have HBsAg testing, regardless of the record of vaccination. If HBsAg is negative and a hepatitis B series has been initiated (and documented), the series should be completed according to the ACIP schedule. If the last recorded dose of hepatitis B vaccine was given less than 30 days before the refugee receives screening for hepatitis B infection, the HBsAg result might reflect vaccine antigen and the test should be repeated. The immunization section of the domestic refugee medical screening guidelines contains more information on specific issues of vaccination during the new arrival refugee screening examination.
Clinical and public health management of persons with HBV infection
Persons with chronic HBV infection should be evaluated by a physician who is experienced and knowledgeable in the management of hepatitis B infection and liver disease. Certain patients with chronic HBV infection will benefit from antiviral therapy, while others will require regular screening for disease progression. Culturally sensitive patient education should be provided, including materials in the refugee’s primary language. Patient education materials for hepatitis infection developed by other agencies may be found at:
- Refugee Health Information Network
- ECHO TV: Hepatitis B
- The Refugee Patient Education Project of CNY
- Refugee Health Technical Assistance Center
Persons who are HBsAg-positive are infected with HBV and should be considered infectious. Because hepatitis B is a reportable disease in some states, cases should be reported to the State or local health department, according to State reporting requirements.
Hepatitic C Virus (HCV)
Background
Hepatitis C is a chronic, generally asymptomatic, form of viral hepatitis that can lead to cirrhosis and hepatocellular carcinoma. The prevalence of hepatitis C virus (HCV) varies between regions and countries (Figure 4), and an estimated 170 million people, or more than 3% of the world’s population, are infected. HCV is transmitted through exposure to infected blood and/or other body fluids. In developed countries, infection primarily results from injection drug use. However, in developing countries, HCV is predominantly transmitted in medical settings where needles may be reused or through transfusions with infected blood products. Although transmission can occur through sexual contact, in the perinatal period, and through breastfeeding, these modes of transmission are inefficient and uncommon.
Overall, 70%-85% of HCV-infected persons will develop liver disease. Several factors may accelerate the progression of chronic hepatitis C, particularly moderate to high alcohol intake. Infection at an older age is also associated with faster progression, as is co-infection with HIV.
Epidemiology of HCV infection in refugee populations
Little generalizable data are available on the epidemiology of HCV infection in refugee populations. Published prevalence rates of anti-HCV antibody have ranged from 0.1% of 1005 Kurdish refugees in Italy in 2003 to 8% of 234 Cambodian refugees in Australia in 2005 3, 4. Prevalence data of HCV infection in refugees currently arriving in the United States are even more sparse. Recently, serum specimens tested from 4,890 Bhutanese, Burmese, Iraqi, and Hmong refugees detected HCV RNA in 63 (1.3%) overall. However, the rates varied by population, with all populations below 1% except for Hmong refugees born in Thailand, whose rate was approximately 7% (unpublished data, personal communication Tonya Mixson-Hayden MD). A recent study published by Shire AM et al. found higher than expected rates of hepatocellular carcinoma in Somali refugees compared with the general U.S. population. In this study, beyond the expected association of hepatocellular carcinoma with chronic HBV, there was also an independent association with chronic HCV infection 5. Therefore, prevalence rates range widely between specific refugee populations from very low (<1%) to very high (7%-8%). Further research is needed to define hepatitis C infection rates in refugee populations.
Recommendations for screening refugees for chronic HCV infection
Adults
Routine screening for HCV infection for refugees during the new arrival medical examination is the same as the guidelines for the general U.S. population. This includes routine screening for those born during 1945-1965 and those with risk factors 6. Identified risk factors or co-infections that should prompt testing in newly arrived refugees include:
- Persons who have ever injected illegal drugs
- Persons who are HIV positive
- Persons who received whole blood or blood components prior to migration
- Persons with other risk factors, such as chronic hemodialysis, persistently abnormal ALT levels, and other risk factors noted in the Recommendations for the Identification of Chronic Hepatitis C Virus Infection Among Persons Born During 1945-1965
Children <18 years of age
CDC does not recommend HCV screening for refugee children during the new arrival medical examination unless they are members of high-risk groups, which include:
- all biological children of HCV-positive mothers and
- children with the same risk factors listed above for adults
With quickly evolving and improving therapy the prevalence threshold for when it is cost-effective to screen a population for HCV infection is currently unknown with experts recommending population rates ranging from 0.8% to 3% 7, 8. Most data indicate that HCV infection rates in refugee populations are below that found in the United States. However, if data become available indicating excessive rates in certain populations, further targeted population screening may be recommended.
Other risk factors in refugee populations may not have been identified, such as female genital cutting with contaminated instruments and tattoos. Additional risk factors may be indications for HCV screening. Visit CDC’s website for updated information on HCV infection.
Testing of low-risk populations is discouraged; such testing increases the likelihood of false-positive anti-HCV screening test results. Further research defining the actual HCV infection rates of subpopulations of refugees is needed and should be encouraged, since such studies can help target screening efforts.
Screening tests that can be used are antibody to HCV (anti-HCV), recombinant immunoblot assay (RIBA), or HCV RNA polymerase chain reaction (PCR). Immunocompromised persons, such as those infected with HIV, those who have end-stage renal disease, and those on immunosuppresive therapy, may have false-negative tests and should receive screening by HCV RNA testing.
Clinical and public health management of chronic HCV infection
Persons who have a positive anti-HCV antibody test should have infection confirmed with HCV RNA testing. Those found to be infected should be managed by a health care professional experienced and knowledgeable in the management of chronic liver disease and hepatitis C. Certain patients with chronic HCV infection (particularly with specific genotypes) will benefit from treatment with antiviral therapy. Culturally appropriate patient education should be conducted, and materials should be provided in the refugee’s primary language when possible.
Hepatitis D Virus (HDV)
Background
Hepatitis D virus (HDV) is a defective virus that requires the presence of HBV to be viable in the human host. It may be acquired as a co-infection with HBV or as a superinfection after hepatitis B infection. An estimated 5% of the world’s 300 million chronic HBV-infected persons are co-infected with HDV. Chronic HBV carriers who are superinfected with HDV usually also develop chronic HDV infection. Progression to cirrhosis and hepatocellular carcinoma is believed to be more common with HBV/HDV co-infections than with HBV infection alone. Identified risk groups include
- Men who have sex with men
- Hemodialysis patients
- Persons with infected sexual contacts
- Injection drug users
- Health care and public safety workers
- Infants born to infected mothers (extremely rare)
The epidemiology of chronic HDV infection in refugees is unknown.
Recommendations for screening refugees for HDV infection
Routine screening is not recommended since HDV has not been reported to infect or cause disease in the absence of HBV infection. The treatment of infection is the same as treatment for hepatitis B.
Hepatitis B vaccination is a primary preventive measure that protects against HDV infection.
Acute Viral Hepatitis
Hepatitis A Virus (HAV)
Background
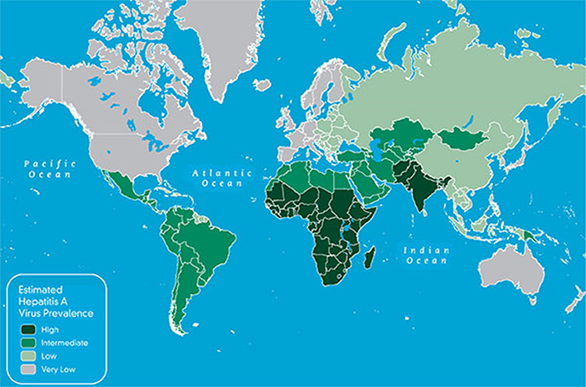
Hepatitis A virus (HAV) is the most common etiology of acute hepatitis. It is endemic in most parts of the world (Figure 5). Most refugees resettling to the United States are from areas that are highly endemic for HAV. HAV is transmitted via the fecal-oral route, and infection is associated with poor sanitation and hygiene. In highly endemic areas, most persons are infected as children. Most refugees resettling to the United States, especially adults, are immune to HAV infection due to previous exposure; resolved HAV infection results in lifetime immunity and does not lead to chronic infection.
Young children with HAV infection typically are asymptomatic and can spread the virus for up to several months. The risk of spread to the general United States population from these children is unknown but appears to be low. Outbreaks have been associated with asymptomatic internationally adopted children placed in nonimmune populations (e.g., host families, day care). Refugees, unlike international adoptees, tend initially to live together following migration, decreasing the potential for an spread outside the refugee community.
Recommendations for screening refugees for HAV infection
Routine testing for HAV infection is not recommended. Persons with signs or symptoms of acute HAV infection (jaundice, abdominal pain, vomiting, elevated liver enzymes) should be evaluated for acute HAV infection with anti-HAV IgM.
Screening for asymptomatic infection in children is expensive, and because a high proportion of refugees are immune, CDC does not recommend routine testing for acute hepatitis A infection in asymptomatic refugees.
Although ideally hepatitis A vaccine (at least the initial dose) should be administered to refugees prior to migration to the United States, currently refugee populations do not receive pre-departure hepatitis A vaccination.
In the United States, the 2-dose hepatitis A vaccination series is recommended for all children 12-23 months of age. All refugee children ages 12-23 months should receive the first dose of hepatitis A vaccine, followed by a second dose 6-18 months later. To ensure immunity for any person over 2 years of age, administer 2 doses of vaccine separated by 6-18 months. In those older than 2 years, prevaccination screening for immunity to hepatitis A (total anti-HAV) among refugees may be cost-effective, depending on the prevalence of hepatitis A infection in the population and local costs for screening 9.
For more information on HAV vaccine recommendations, see the immunization guidelines section for newly arrived refugees; the ACIP vaccination guidelines [PDF – 21 page] for the United States; and updated hepatitis A vaccination guidance from the ACIP.
Hepatitis E Virus (HEV)
Background
Hepatitis E virus (HEV) causes acute viral hepatitis and is transmitted by the fecal-oral route. The signs and symptoms are similar to those produced by HAV. HEV infection is well described in many resource-limited nations, particularly in South Asia, although its prevalence is likely underestimated, including in developed nations 10. Although fulminant hepatitis may develop in 0.5%-4% of the overall HEV-infected population, it is particularly virulent in pregnant women, especially those infected during the third trimester, when mortality rates of up to 25% have been reported. The incubation period of HEV ranges from 3 to 8 weeks, with a mean of 40 days. Clinical presentation and epidemiologic characteristics vary by genotype. Although chronic infection has not been reported among persons infected with genotype 1 and 2 (the strain commonly causing disease in developing countries), infection with HEV genotype 3 has been associated with chronic hepatitis. Recent reports suggest that the rates of acute HEV infection are increasing and may be chronic among solid organ transplant recipients, persons with hematologic malignancy, and HIV-positive patients 11. Only persons with signs or symptoms of acute hepatitis should be tested for HEV infection along with HAV, HBV, and HCV infection, since these viruses are clinically indistinguishable. The diagnosis is confirmed by detection of RNA in the blood by reverse transcriptase-polymerase chain reaction.
Recommendations for screening refugees for HEV infection
Routine testing for HEV infection is not recommended for refugees of any age.
Figures
Figure 1: Geographic distribution of chronic hepatitis B virus (HBV) Infection – worldwide, 2006*
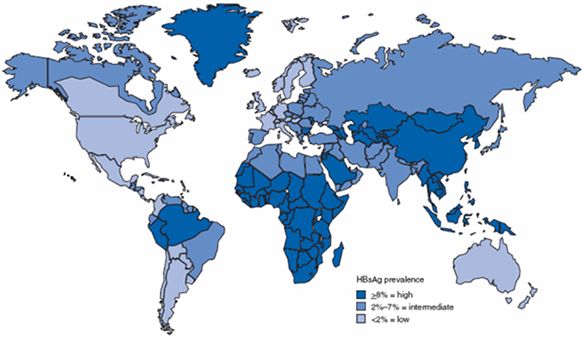
* For multiple countries, estimates of prevalence of hepatitis B surface antigen (HBsAg), a marker of chronic HBV Infection, are based on limited data and might not reflect current prevalence in countries that have implemented childhood hepatitis B vaccination. In addition, HBsAg prevalence might vary within countries by subpopulation and locality.
Source: CDC. Travelers' health; yellow book. Atlanta, GA: US Department of Health and Human Services, CDC; 2008.
Figure 2: Hepatitis B screening algorithm for those ≥ 18 years old born in countries with hepatitis B prevalence rates of ≥ 2%, or those considered at high risk in countries where prevalence rates are < 2%
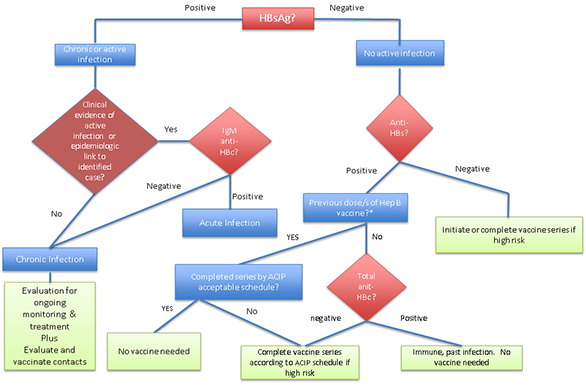
*Many refugees have initiated hepatitis B vaccine series but will not have completed by the time of arrival
Figure 3: Hepatitis B screening algorithm for those < 18 years old born in countries with hepatitis B prevalence rates of ≥ 2
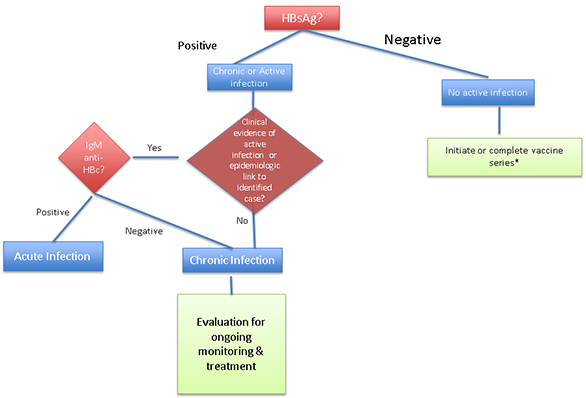
*checking anti-HBs and total anti-HBc, as in adults, may be reasonable in older children in populations with high prevalence rates or who are at risk of infection.
Figure 4: Geographic Prevalence of Hepatitis C Virus Infection
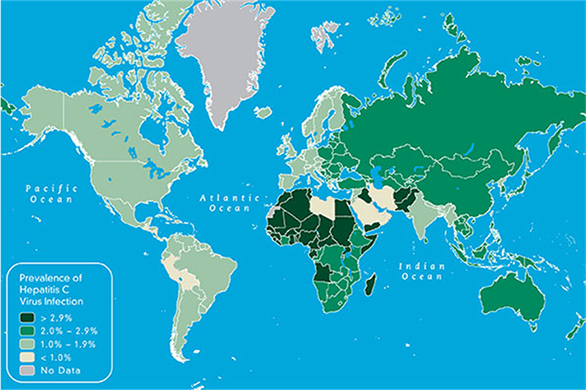
Figure 5: Estimated prevalence of hepatitis A virus, 2005
References
- Kloster B, Kramer R, Eastlund T, Grossman B, Zarvan B. Hepatitis B surface antigenemia in blood donors following vaccination. Transfusion 1995;35:475—7.
- Lunn ER, Hoggarth BJ, Cook WJ. Prolonged hepatitis B surface antigenemia after vaccination. Pediatrics 2000;105:E81.
- Tafuri S, Prato R, Martinelli D, et al. Prevalence of Hepatitis B, C, HIV and syphilis markers among refugees in Bari, Italy. BMC Infectious Diseases 2010;10:213.
- Caruna SR, Kelly HA, De Silva SL, et. al. Knowledge about hepatitis and previous exposure to hepatitis viruses in immigrants and refugees from the Mekong Region. Aust N Z J Public Health 2005;29(1):64-8.
- Shire AM, Sanchu DS, Kaiya JK, et al. Viral hepatitis among Somali immigrants in Minnesota: Association of hepatitis C with hepatocellular carcinoma. Mayo Clin Proc 2012;87(1):17-24.
- Greenaway C, Wong DKH, Assayag D, et al. Screening for hepatitis C infection: evidence review for arriving immigrants and refugees. Appendix 7. Guidelines for Immigrant Health. Canadian Medical Association Journal. 2010 0:cmaj.090313v1; doi:10.1503/cmaj.090313.
- Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Morb Mort Wkly Rep 2012;61(RR04):1-18.
- Eckman MH, Tala AH, Gordon SC, et al. Cost-effectiveness of screening for chronic hepatitis C infection in the United States. Clin Infect Dis 2013; doi: 10.1093/cid/cit069.
- Barnett ED. Immunizations and infectious disease screening for internationally adopted children. Pediatr Clin North Am. 2005 Oct;52(5):1287–309, vi.
- Drobeniuc J, Greene-Montfort T, Le NT, et al. Hepatitis E in the United States: Results from a laboratory-based surveillance 2005-2012. Emerg Infect Dis 2013;19(2):218-222.
- Dalton HR, Bendall RP, Keane FE, Tedder RS. Persistent carriage of hepatitis E in patients with HIV infection. N Engl J Med 2009;361:1025-1027.
- Page last reviewed: March 6, 2014
- Page last updated: March 6, 2014
- Content source:



 ShareCompartir
ShareCompartir