Histopathology
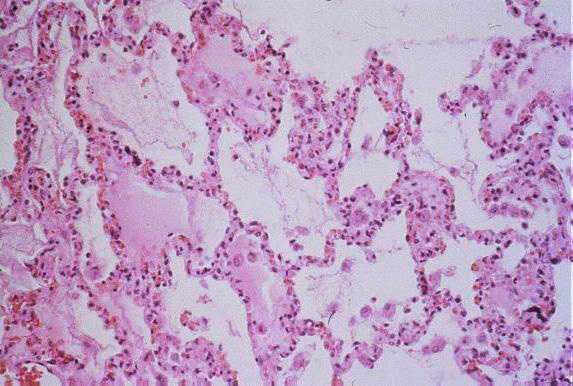
Low-power photomicrograph showing interstitial pneumonitis and intraalveolar edema (Click image for larger view) Image courtesy Sherif R. Zaki, M.D., Ph.D.
No single pathognomonic lesion is found that would permit certain histopathologic diagnosis of HPS. In fact, the incipient stages of ARDS can create a picture of pulmonary edema similar to HPS. However, the total picture is rather distinctive. Pathology in HPS patients is characterized mainly by pulmonary findings, as well as findings in the spleen, liver, and lymph nodes.
Grossly, the lungs are dense, rubbery and heavy, usually weighing twice as much as the average lung. They are often found floating in a pool of yellow serous fluid within the pleural cavity.
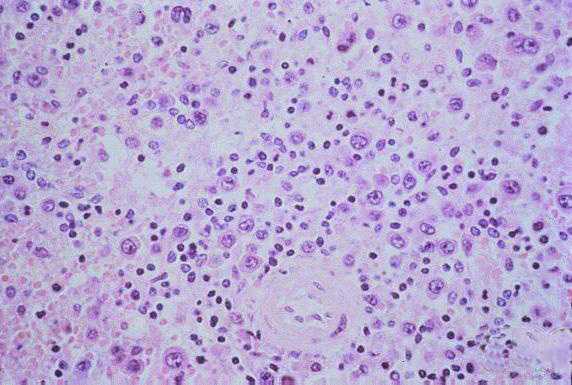
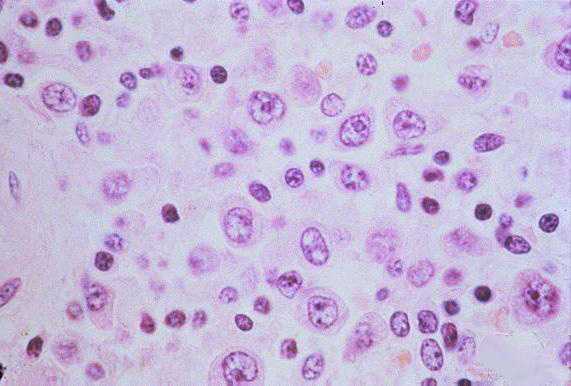
The pathologic lesions are primarily vascular with variable degrees of generalized capillary dilation and edema. Morphologic changes of the endothelium are uncommon and, when present, consist of prominent and swollen endothelial cells. Histopathologic lesions are mainly seen in the lung and spleen. In most cases, the lungs reveal a mild to moderate interstitial pneumonitis with variable degrees of congestion, edema, and mononuclear cell infiltration. The cellular infiltrate is composed of small and enlarged mononuclear cells with the appearance of immunoblasts. Focal hyaline membranes are observed, as well as extensive intraalveolar edema and fibrin. Neutrophils are scanty, and the respiratory epithelium is intact in typical cases, with no evidence of cellular debris, nuclear fragmentation, or type II pneumocyte hyperplasia.
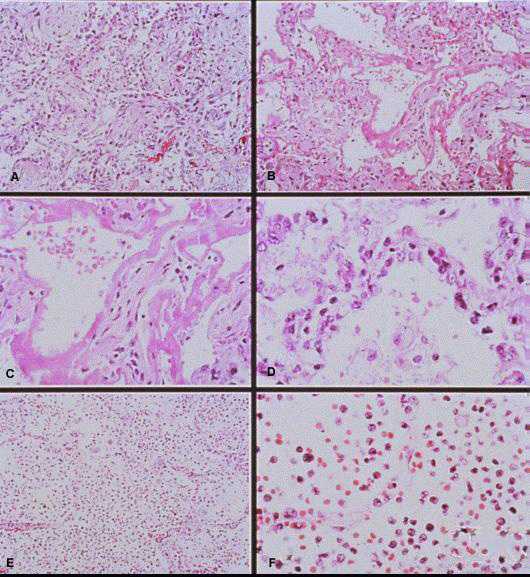
Photomicrographs showing histopathological features less commonly seen in cases of HPS.
A: Lung showing extensive interstitial and alveolar fibrosis. Noe the increased interstitial cellularity with numerous fibroblasts. Original magnification: x50
B: Patchy areas of alveolar septal thickening and prominent hyaline membranes. Original magnification: x50
C: Higher magnification showing typical dense laminated hyaline membranes. Original magnification: x100
D: Alveolar septum showing prominent type II pneumocyte proliferation. Original magnification: x158
E: Abundant polymorphonuclear leukocytes fill alveolar spaces with focal destruction of alveolar septa. Original magnification: x50
F: Higher power magnification showing the antraalveolar exudate composed mainly of polymorphonuclear leukocytes, red blood cells, and fibrin. Original magnification: x158
(Click image for larger view)
Images courtesy of Sherif R. Zaki, M.D., Ph.D.
Among patients who die after a longer-than-average course of the disease, and in lung biopsy specimens from survivors, the histopathologic changes are more characteristic of exudative and proliferative stages of diffuse alveolar damage. In these cases, proliferation of reparative type II pneumocytes, severe edematous and fibroblastic thickening of the alveolar septa with severe airspace disorganization, and distortion of lung architecture can be seen.
Other typical histopathologic findings are seen in lymphoid tissues of HPS patients. These include the presence of immunoblasts within the red pulp and periarteriolar sheaths of the spleen and paracortex, within sinuses of lymph nodes, and in the peripheral blood.
- Page last reviewed: August 29, 2012
- Page last updated: August 29, 2012
- Content source:


 ShareCompartir
ShareCompartir