Diagnostics
A positive serological test result, evidence of viral antigen in tissue by immunohistochemistry, or the presence of amplifiable viral RNA sequences in blood or tissue, with compatible history of HPS, is considered diagnostic for HPS.
Serologic Assays
At the time of the 1993 outbreak in the Four Corners area, cross-reactive antibodies to the previously known hantaviruses (e.g., Hantaan, Seoul, Puumala, and Prospect Hill viruses) were found in the acute- and convalescent-phase sera of some of the initial HPS patients. Tests based on specific viral antigens from SNV have since been developed and are now widely used for the routine diagnosis of HPS. CDC uses an enzyme-linked immunosorbent assay (ELISA) to detect IgM antibodies to SNV and to diagnose acute infections with other hantaviruses. This assay is also available in some state health laboratories.
An IgG test is used in conjunction with the IgM-capture test. Acute- and convalescent-phase sera should reflect a four-fold rise in IgG antibody titer or the presence of IgM in acute-phase sera to be diagnostic for hantaviral disease. Note that acute-phase serum sent as an initial diagnostic specimen may not yet have IgG. IgG antibody is long lasting, and sera of patients retrospectively identified appear to have retained antibody for many years. The SNV IgG ELISA has therefore been used in serologic investigations of the epidemiology of the disease and appears to be appropriate for this purpose. Investigations of selected populations using this assay have confirmed that infections with the virus are not common and that mild or inapparent infections are rare.
A Western blot assay using recombinant antigens and isotype-specific conjugates for IgM-IgG differentiation has also been developed and its results are generally in agreement with those of the IgM-capture format.
Also in use is a rapid immunoblot strip assay (RIBA), an investigational prototype assay to identify serum antibody to recombinant proteins and peptides specific for SNV and other hantaviruses.
Serologic confirmation of hantaviral infections has traditionally been done with neutralizing plaque assays, which have been recently described for SNV. However, these specific assays are also not commercially available.
Isolation
Isolation of hantaviruses (see below) from human sources is difficult, and the viruses causing HPS seem to be no exception to this rule. To date, no isolates of SNV-like viruses have been recovered from humans, and therefore virus isolation is not a consideration for diagnostic purposes.
Immunohistochemistry (IHC)
IHC testing of formalin-fixed tissues with specific monoclonal and polyclonal antibodies can be used to detect hantavirus antigens and has proven to be a sensitive method for laboratory confirmation of hantaviral infections. IHC has an important role in the diagnosis of HPS in patients from whom serum samples and frozen tissues are unavailable for diagnostic testing and in the retrospective assessment of disease prevalence in a defined geographic region.
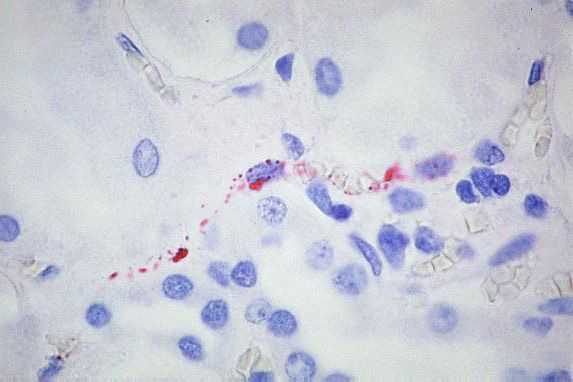
High power magnification showing immunostaining of renal interstitial capillaries using Peromyscus serum (Click image for larger view)
Image courtesy of Sherif R. Zaki, M.D., Ph.D.
Polymerase Chain Reaction (PCR)
Reverse transcriptase-PCR (RT-PCR) can be used to detect hantaviral RNA in fresh frozen lung tissue, blood clots, or nucleated blood cells. However, RT-PCR is very prone to cross-contamination and should be considered an experimental technique. Differences in viruses in the United States complicate the use and sensitivity of RT-PCR for the routine diagnosis of hantaviral infections.
Related Links
- Page last reviewed: August 29, 2012
- Page last updated: August 29, 2012
- Content source:


 ShareCompartir
ShareCompartir