Interim Duodenoscope Surveillance Protocol
Interim Protocol for Healthcare Facilities Regarding Surveillance for Bacterial Contamination of Duodenoscopes after Reprocessing
Please also see FAQs on the Interim Duodenoscope Surveillance Protocol.
Outbreaks of bacterial infection associated with endoscopes are often attributed to improperly reprocessed endoscopes. However, recent reports have identified carbapenem-resistant Enterobacteriaceae (CRE) transmission associated with persistently contaminated duodenoscopes for which no breaches in reprocessing were identified (1).

There is currently very limited information to guide the use of surveillance cultures to assess endoscope reprocessing outside of recognized outbreak settings. Surveillance cultures are not a replacement for appropriate training and oversight of endoscope reprocessing practices. Before initiating surveillance cultures, facilities considering their use should involve key facility staff, including the clinical laboratory director, clinical staff, infection prevention staff, hospital epidemiologists, and risk management staff to develop a plan for implementation, and response (e.g., patient notification) to surveillance culture results.
The following considerations are intended for facilities that perform procedures using duodenoscopes to assess the adequacy of reprocessing. While these measures apply primarily for duodenoscopes, they can also be implemented for other flexible endoscopes that have an elevator mechanism (e.g., used to perform endoscopic ultrasound). This document is intended to supplement and not replace or modify manufacturer recommended reprocessing procedures. This is an interim protocol and measures outlined below may change as new information becomes available.
Duodenoscope Reprocessing: Facilities should review all steps in duodenoscope reprocessing several times a year (e.g., quarterly) and ensure strict adherence to the manufacturer’s instructions, paying particular attention to the following:
- Inspection and manual cleaning: Ensure that the elevator mechanism located at the distal tip of the duodenoscope is thoroughly cleaned and free of all visible debris. The visible inspection is to be done with the elevator in the “open/raised” position as well as with the elevator in the “closed/lowered” position to ensure there is no visible debris above or below the elevator mechanism. Consideration should be given to use of a magnifying glass (e.g., 10x) to improve detection of residual debris around the elevator mechanism.
- Drying: Ensure that the channels of the duodenoscope and elevator mechanism are thoroughly dried prior to storage. This should include an alcohol flush followed by forced air drying if these procedures are compatible with the duodenoscope per the manufacturer’s instructions. If channels and the elevator mechanism are not completely dry, bacterial growth can occur, forming a biofilm that is difficult to remove and could result in persistent contamination.
- Use of Duodenoscope Culturing
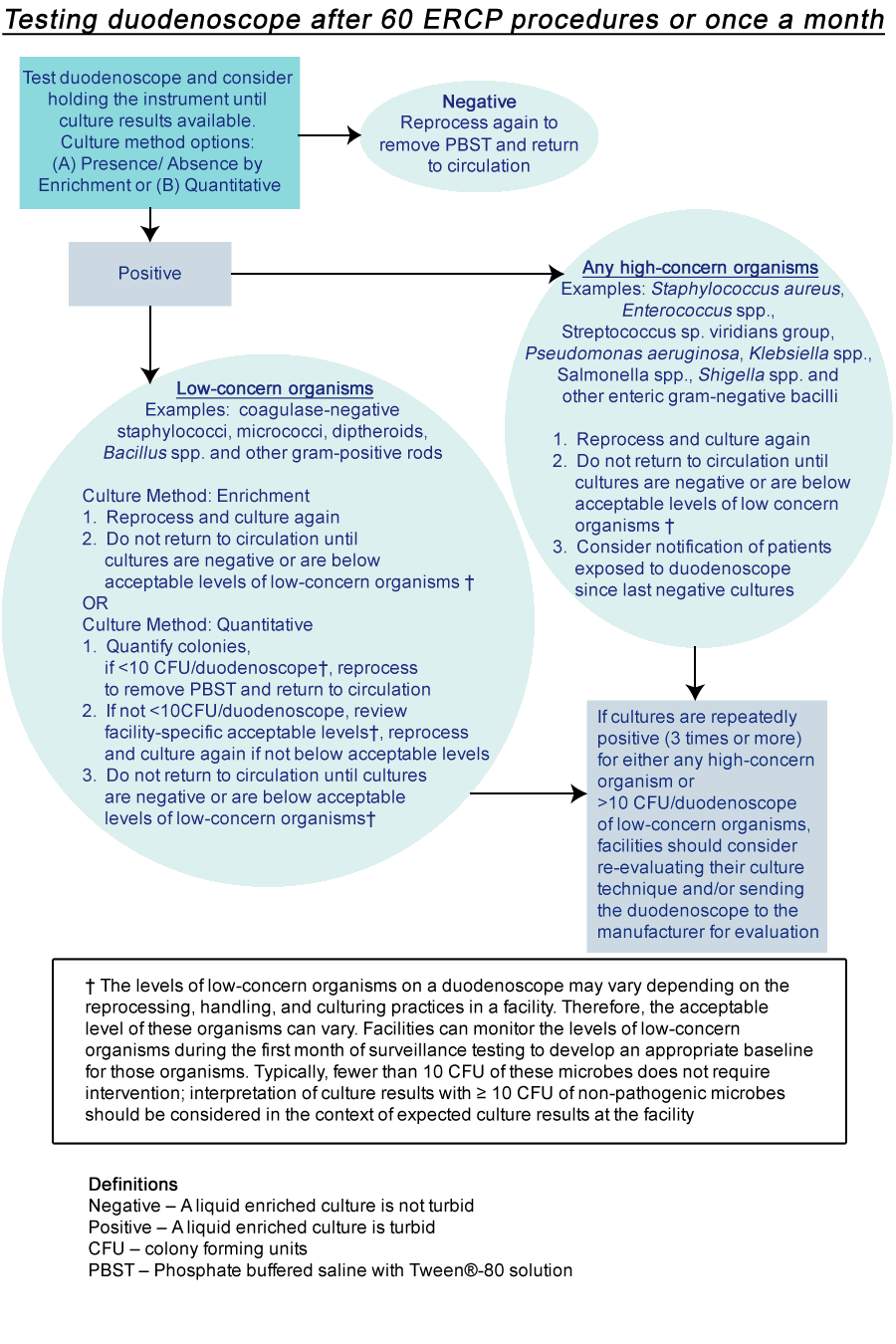
- Surveillance: Although routine culturing of endoscopes is not part of current U.S. guidelines, recent outbreaks associated with duodenoscopes have led some facilities to consider regular monitoring to assess the adequacy of duodenoscope reprocessing (see algorithm).
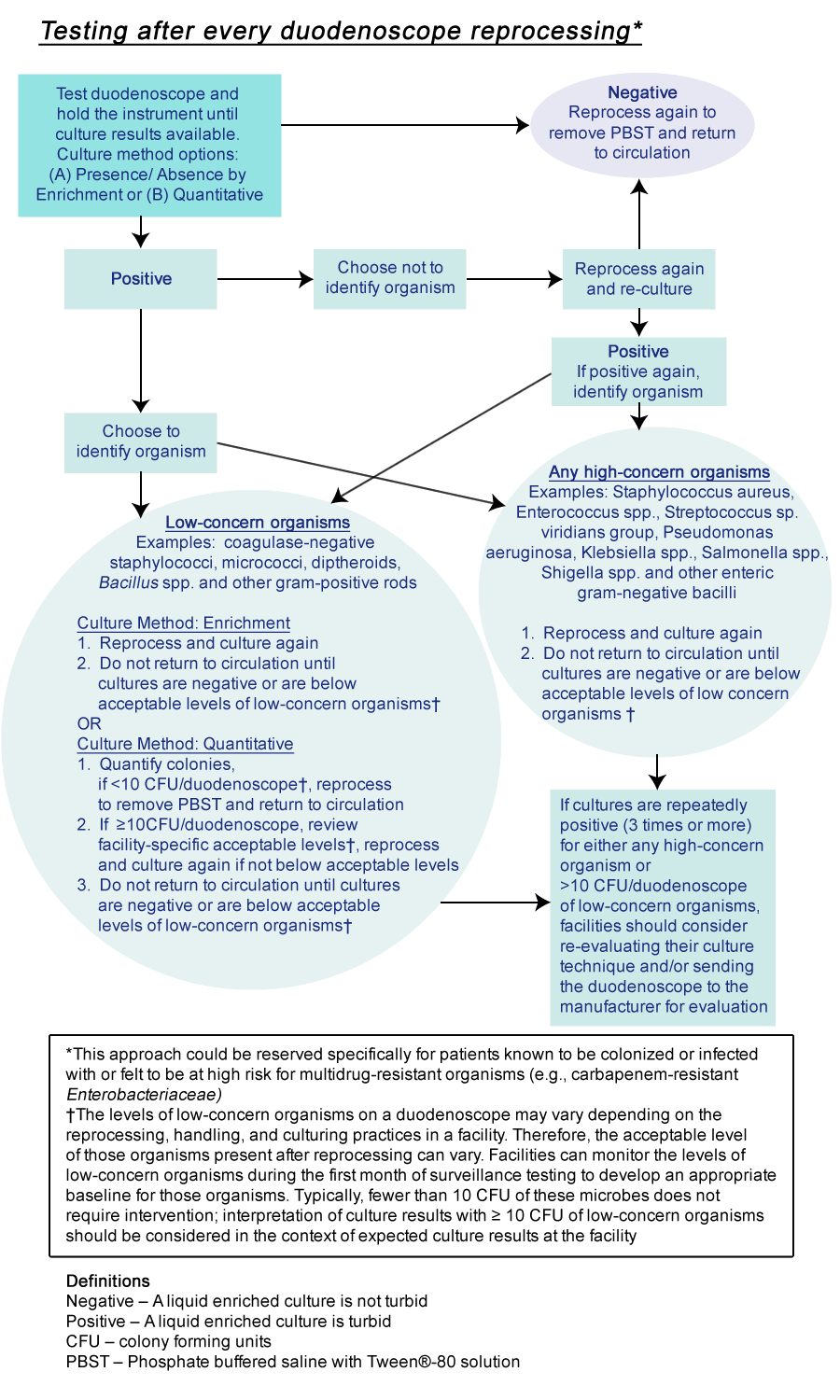
- The optimal frequency of surveillance cultures has not been established. International guidelines have recommended intervals ranging from every 4 weeks to annually (2, 3). A facility choosing to perform surveillance cultures can consider performing post-reprocessing cultures periodically, e.g., monthly or after every 60 procedures for each duodenoscope. Some facilities could choose to perform duodenoscope cultures weekly (e.g., after procedures on Friday to allow cultures to incubate over the weekend). Alternatively, facilities can choose to perform cultures, after reprocessing, following each use.
- Cultures should be obtained after the duodenoscope has been reprocessed (after drying) and should include at least the instrument channel and the distal end of the duodenoscope (i.e., elevator mechanism and elevator recess for duodenoscopes with sealed elevator wire channel; and elevator mechanism, elevator recess, and elevator channel for duodenoscopes with unsealed elevator wire channels). An interim sampling protocol developed by CDC that represents one approach to culturing of duodenoscopes is available at the following link (Interim Duodenoscope Sampling Method and Interim Duodenoscope Culture Method). Facilities may choose other sampling methods (e.g., flush-brush-flush method), or choose to sample additional channels beyond those specified in this approach. The sensitivity of the interim protocol has not been determined. A negative culture does not completely exclude the possibility of a contaminated duodenoscope. However, positive culture results should lead to some action as described below.
- Post-reprocessing cultures of duodenoscopes should be assessed for two types of microbial growth: high- and low-concern organisms. If successfully disinfected, culturing should not detect any high-concern organisms (i.e., organisms more often associated with disease), such as Gram-negative bacteria (e.g., Escherichia coli, Klebsiella pneumoniae or other Enterobacteriaceae, as well as Pseudomonas aeruginosa), Staphylococcus aureus, and Enterococcus. Small numbers of low-concern organisms (i.e., organism less often associated with disease and potentially a result of contamination of cultures during collection) might occasionally be detected (e.g., coagulase-negative staphylococci excluding Staphylococcus lugdunensis, Bacillus species, diphtheroids). The levels of these low-concern contaminants on a duodenoscope can vary depending on the reprocessing, handling, and culturing practices in a facility; levels of such organisms detectable after reprocessing will therefore vary. Facilities can monitor the levels of these bacteria within the first month of surveillance testing to develop an expected baseline for those organisms. Typically, fewer than 10 colony forming unit (CFU) of low-concern microbes does not require intervention; interpretation of culture results with ≥ 10 CFU of low-concern microbes should be considered in the context of typical culture results at the facility. Any quantity of high-concern organism (i.e., one colony or greater) warrants further remedial actions as described below. This is consistent with previous recommendations (2, 4).
- Holding duodenoscopes out of use while surveillance culture results are pending could be considered, especially if performing surveillance cultures after each use. Any duodenoscope found to be contaminated should not be returned to use until steps outlined in remedial actions section (below) are addressed.
- Facilities should ensure that each endoscopic procedure is appropriately documented with regard to the specific endoscope used in order to allow identification of exposed patients should microbial growth be detected as described above. Furthermore, results of post-reprocessing duodenoscope cultures should be logged and tracked for each duodenoscope.
- Non-culture methods (e.g., adenosine triphosphate (ATP) bioluminescence assays) have been used to assess duodenoscope reprocessing by detecting residual organic material after cleaning. While individual facilities might choose to use such non-culture assays, more work is needed to interpret their results since non-culture methods lack consistent correlation to bacterial concentrations. They might, however, provide insight regarding the quality of duodenoscope reprocessing if systematically validated (5, 6).
- Surveillance: Although routine culturing of endoscopes is not part of current U.S. guidelines, recent outbreaks associated with duodenoscopes have led some facilities to consider regular monitoring to assess the adequacy of duodenoscope reprocessing (see algorithm).
- During outbreaks
- Surveillance cultures have been used during outbreaks to identify contaminated duodenoscopes and to ensure that ongoing contamination is not occurring
- Until the limits of detection are defined, negative surveillance cultures alone should not be used to rule out duodenoscopes as a source of cross-transmission.
- Following documented transmission of bacteria via a duodenoscope, facilities should consider performing a series (e.g. 3 to 5) of duodenoscope surveillance cultures after reprocessing to ensure that the interventions employed to address the issue have eliminated contamination and are preventing further contamination that could lead to transmission.
- An interim sampling protocol developed by CDC is available at the following link (Interim Duodenoscope Sampling Method and Interim Duodenoscope Culture Method).
- Remedial Actions:
- Any duodenoscope found to be contaminated with any high-concern organisms or unacceptable CFU of low-concern organisms should be reprocessed again with repeat post-reprocessing cultures obtained. The duodenoscope should not be used again until it has been demonstrated to be free of high-concern organisms and has an acceptable level of low-concern organisms. Positive cultures should prompt a procedure review to ensure adherence to the manufacturer’s reprocessing instructions and to ensure cultures are being performed correctly. If a reprocessing breach is identified, appropriate facility personnel (e.g., infection prevention staff) should be notified and corrective actions should be immediately implemented. Refer to the manufacturer’s instructions for evaluating the duodenoscope for defects when bacteria are persistently recovered by duodenoscope cultures (including repeated cultures positive for low-concern organisms). In this situation, the facility can consider having the duodenoscope evaluated by the manufacturer. In addition, when unsuccessful reprocessing is suspected based on surveillance cultures, it might be helpful to review positive cultures among affected patients to determine whether other clusters of relevant pathogens could have been transmitted.
- Patient Information and Notification: Patients undergoing procedures using duodenoscopes should be informed during the consenting process that there is a risk of patient-to-patient bacterial transmission associated with the procedure, including uncommon transmission of a multidrug-resistant organism. Facilities should document the specific duodenoscope used for each patient to facilitate identification of the exposed patients if needed. If high-concern organisms are recovered from a reprocessed duodenoscope (as described above), the decision to notify patients of their potential exposure should be made in consultation with key facility staff, including involved healthcare providers, infection prevention staff, hospital epidemiologists, and risk management. In instances where a multidrug-resistant organism (e.g., CRE) is cultured from a reprocessed duodenoscope, screening of exposed patients for the organism should be considered (a laboratory protocol for rectal CRE screening is available in the CDC CRE toolkit [PDF – 110KB]). This allows for appropriate infection control precautions to be implemented during future admissions to a healthcare facility for any exposed patient with positive screening cultures for the multidrug-resistant organism. Detailed information is available at patient notifications.
- Staff Training and Competency: Ensure personnel performing reprocessing of duodenoscopes have received appropriate training with competency verification for reprocessing procedures. Competencies should be assessed at initiation of employee duties and at least annually and anytime a breach is identified or when a new technique or equipment is introduced. Competency verification should include direct observation in addition to other assessments per facility policy (e.g., written tests). Personnel responsible for reprocessing endoscopes are encouraged to seek certification in flexible endoscope reprocessing.
References
- Epstein L, Hunter JC, Arwady MA, et al. New Delhi Metallo-β-Lactamase Producing Carbapenem-Resistant Escherichia coli Associated with Exposure to Duodenoscopes. JAMA 2014;312:1447-1455
- Queensland Government, Queensland Health. Endoscope Reprocessing, Section 6.4 Microbiological Testing. Available at: http://www.health.qld.gov.au/EndoscopeReprocessing/module_6/6_4.asp
- Beilenhoff U, Neumann CS, Rey JF, Biering H, Blum R, Schmidt V and ESGE Guidelines Committee. ESGE-ESGENA guideline for quality assurance in reprocessing: microbiological surveillance testing in endoscopy. Endoscopy. 2007;39:175-81.
- CDC / Healthcare Infection Control Practices Advisory Committee. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Available at: https://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf [PDF – 950 KB]
- Alfa MJ, Fatima I, Olson N. The adenosine triphosphate test is a rapid and reliable audit tool to assess manual cleaning adequacy of flexible endoscope channels. Am J Infect Control 2013;41:294-53
- Hansen D, Benner D, Hilgenhöner M, Leisebein T, Brauksiepe A, Popp W. ATP measurement as method to monitor the quality of reprocessing flexible endoscopes. Ger Med Sci. 2004;2:Doc04.
Click here for decription of the above image [Txt – 6 KB]
- Page last reviewed: March 11, 2015
- Page last updated: April 3, 2015
- Content source:


 ShareCompartir
ShareCompartir
