Olfaction
Olfaction is a chemoreception that forms the sense of smell. Olfaction has many purposes, such as the detection of hazards, pheromones, and food. It integrates with other senses to form the sense of flavor.[1]
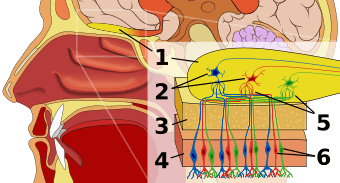
Olfaction occurs when odorants bind to specific sites on olfactory receptors located in the nasal cavity.[2] Glomeruli aggregate signals from these receptors and transmit them to the olfactory bulb, where the sensory input will start to interact with parts of the brain responsible for smell identification, memory, and emotion.[3]
Often, land organisms will have separate olfaction systems for smell and taste (orthonasal smell and retronasal smell), but water-dwelling organisms usually have only one system.[4]
Olfactory dysfunction arises as the result of many different peripheral and central disturbances, including upper respiratory infections, traumatic brain injury, and neurodegenerative disease. [5] [6]
History

Early scientific study of olfaction includes the extensive doctoral dissertation of Eleanor Gamble, published in 1898, which compared olfactory to other stimulus modalities, and implied that smell had a lower intensity discrimination.[7] As the Epicurean and atomistic Roman philosopher Lucretius (1st century BCE) speculated, different odors are attributed to different shapes and sizes of "atoms" (odor molecules in the modern understanding) that stimulate the olfactory organ . A modern demonstration of that theory was the cloning of olfactory receptor proteins by Linda B. Buck and Richard Axel (who were awarded the Nobel Prize in 2004), and subsequent pairing of odor molecules to specific receptor proteins. Each odor receptor molecule recognizes only a particular molecular feature or class of odor molecules. Mammals have about a thousand genes that code for odor reception.[8] Of the genes that code for odor receptors, only a portion are functional. Humans have far fewer active odor receptor genes than other primates and other mammals.[9] In mammals, each olfactory receptor neuron expresses only one functional odor receptor.[10] Odor receptor nerve cells function like a key–lock system: if the airborne molecules of a certain chemical can fit into the lock, the nerve cell will respond. There are, at present, a number of competing theories regarding the mechanism of odor coding and perception. According to the shape theory, each receptor detects a feature of the odor molecule. The weak-shape theory, known as the odotope theory, suggests that different receptors detect only small pieces of molecules, and these minimal inputs are combined to form a larger olfactory perception (similar to the way visual perception is built up of smaller, information-poor sensations, combined and refined to create a detailed overall perception). According to a new study, researchers have found that a functional relationship exists between molecular volume of odorants and the olfactory neural response.[11] An alternative theory, the vibration theory proposed by Luca Turin,[12][13] posits that odor receptors detect the frequencies of vibrations of odor molecules in the infrared range by quantum tunnelling. However, the behavioral predictions of this theory have been called into question.[14] There is no theory yet that explains olfactory perception completely.
Main olfactory system
In vertebrates, smells are sensed by olfactory sensory neurons in the olfactory epithelium. The olfactory epithelium is made up of at least six morphologically and biochemically different cell types.[15] The proportion of olfactory epithelium compared to respiratory epithelium (not innervated, or supplied with nerves) gives an indication of the animal's olfactory sensitivity. Humans have about 10 cm2 (1.6 sq in) of olfactory epithelium, whereas some dogs have 170 cm2 (26 sq in). A dog's olfactory epithelium is also considerably more densely innervated, with a hundred times more receptors per square centimeter.[16]
Molecules of odorants passing through the superior nasal concha of the nasal passages dissolve in the mucus that lines the superior portion of the cavity and are detected by olfactory receptors on the dendrites of the olfactory sensory neurons. This may occur by diffusion or by the binding of the odorant to odorant-binding proteins. The mucus overlying the epithelium contains mucopolysaccharides, salts, enzymes, and antibodies (these are highly important, as the olfactory neurons provide a direct passage for infection to pass to the brain). This mucus acts as a solvent for odor molecules, flows constantly, and is replaced approximately every ten minutes.
In insects, smells are sensed by olfactory sensory neurons in the chemosensory sensilla, which are present in insect antenna, palps, and tarsa, but also on other parts of the insect body. Odorants penetrate into the cuticle pores of chemosensory sensilla and get in contact with insect odorant-binding proteins (OBPs) or Chemosensory proteins (CSPs), before activating the sensory neurons.
Receptor neuron
The binding of the ligand (odor molecule or odorant) to the receptor leads to an action potential in the receptor neuron, via a second messenger pathway, depending on the organism. In mammals, the odorants stimulate adenylate cyclase to synthesize cAMP via a G protein called Golf. cAMP, which is the second messenger here, opens a cyclic nucleotide-gated ion channel (CNG), producing an influx of cations (largely Ca2+ with some Na+) into the cell, slightly depolarising it. The Ca2+ in turn opens a Ca2+-activated chloride channel, leading to efflux of Cl−, further depolarizing the cell and triggering an action potential. Ca2+ is then extruded through a sodium-calcium exchanger. A calcium-calmodulin complex also acts to inhibit the binding of cAMP to the cAMP-dependent channel, thus contributing to olfactory adaptation.
The main olfactory system of some mammals also contains small subpopulations of olfactory sensory neurons that detect and transduce odors somewhat differently. Olfactory sensory neurons that use trace amine-associated receptors (TAARs) to detect odors use the same second messenger signaling cascade as do the canonical olfactory sensory neurons.[17] Other subpopulations, such as those that express the receptor guanylyl cyclase GC-D (Gucy2d)[18] or the soluble guanylyl cyclase Gucy1b2,[19] use a cGMP cascade to transduce their odorant ligands.[20][21][22] These distinct subpopulations (olfactory subsystems) appear specialized for the detection of small groups of chemical stimuli.
This mechanism of transduction is somewhat unusual, in that cAMP works by directly binding to the ion channel rather than through activation of protein kinase A. It is similar to the transduction mechanism for photoreceptors, in which the second messenger cGMP works by directly binding to ion channels, suggesting that maybe one of these receptors was evolutionarily adapted into the other. There are also considerable similarities in the immediate processing of stimuli by lateral inhibition.
Averaged activity of the receptor neurons can be measured in several ways. In vertebrates, responses to an odor can be measured by an electro-olfactogram or through calcium imaging of receptor neuron terminals in the olfactory bulb. In insects, one can perform electroantennography or calcium imaging within the olfactory bulb.
Olfactory bulb projections
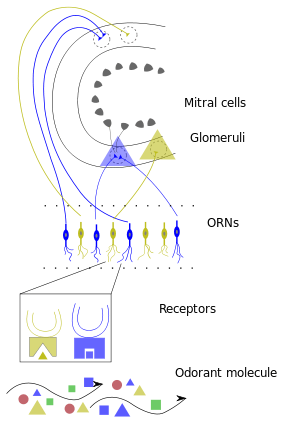
Olfactory sensory neurons project axons to the brain within the olfactory nerve, (cranial nerve I). These nerve fibers, lacking myelin sheaths, pass to the olfactory bulb of the brain through perforations in the cribriform plate, which in turn projects olfactory information to the olfactory cortex and other areas.[23] The axons from the olfactory receptors converge in the outer layer of the olfactory bulb within small (≈50 micrometers in diameter) structures called glomeruli. Mitral cells, located in the inner layer of the olfactory bulb, form synapses with the axons of the sensory neurons within glomeruli and send the information about the odor to other parts of the olfactory system, where multiple signals may be processed to form a synthesized olfactory perception. A large degree of convergence occurs, with 25,000 axons synapsing on 25 or so mitral cells, and with each of these mitral cells projecting to multiple glomeruli. Mitral cells also project to periglomerular cells and granular cells that inhibit the mitral cells surrounding it (lateral inhibition). Granular cells also mediate inhibition and excitation of mitral cells through pathways from centrifugal fibers and the anterior olfactory nuclei. Neuromodulators like acetylcholine, serotonin and norepinephrine all send axons to the olfactory bulb and have been implicated in gain modulation,[24] pattern separation,[25] and memory functions,[26] respectively.
The mitral cells leave the olfactory bulb in the lateral olfactory tract, which synapses on five major regions of the cerebrum: the anterior olfactory nucleus, the olfactory tubercle, the amygdala, the piriform cortex, and the entorhinal cortex. The anterior olfactory nucleus projects, via the anterior commissure, to the contralateral olfactory bulb, inhibiting it. The piriform cortex has two major divisions with anatomically distinct organizations and functions. The anterior piriform cortex (APC) appears to be better at determining the chemical structure of the odorant molecules, and the posterior piriform cortex (PPC) has a strong role in categorizing odors and assessing similarities between odors (e.g. minty, woody, and citrus are odors that can, despite being highly variant chemicals, be distinguished via the PPC in a concentration-independent manner).[27] The piriform cortex projects to the medial dorsal nucleus of the thalamus, which then projects to the orbitofrontal cortex. The orbitofrontal cortex mediates conscious perception of the odor (citation needed). The three-layered piriform cortex projects to a number of thalamic and hypothalamic nuclei, the hippocampus and amygdala and the orbitofrontal cortex, but its function is largely unknown. The entorhinal cortex projects to the amygdala and is involved in emotional and autonomic responses to odor. It also projects to the hippocampus and is involved in motivation and memory. Odor information is stored in long-term memory and has strong connections to emotional memory. This is possibly due to the olfactory system's close anatomical ties to the limbic system and hippocampus, areas of the brain that have long been known to be involved in emotion and place memory, respectively.
Since any one receptor is responsive to various odorants, and there is a great deal of convergence at the level of the olfactory bulb, it may seem strange that human beings are able to distinguish so many different odors. It seems that a highly complex form of processing must be occurring; however, as it can be shown that, while many neurons in the olfactory bulb (and even the pyriform cortex and amygdala) are responsive to many different odors, half the neurons in the orbitofrontal cortex are responsive to only one odor, and the rest to only a few. It has been shown through microelectrode studies that each individual odor gives a particular spatial map of excitation in the olfactory bulb. It is possible that the brain is able to distinguish specific odors through spatial encoding, but temporal coding must also be taken into account. Over time, the spatial maps change, even for one particular odor, and the brain must be able to process these details as well.
Inputs from the two nostrils have separate inputs to the brain, with the result that, when each nostril takes up a different odorant, a person may experience perceptual rivalry in the olfactory sense akin to that of binocular rivalry.[28]
In insects, smells are sensed by sensilla located on the antenna and maxillary palp and first processed by the antennal lobe (analogous to the olfactory bulb), and next by the mushroom bodies and lateral horn.
Accessory olfactory system
Many animals, including most mammals and reptiles, but not humans, have two distinct and segregated olfactory systems: a main olfactory system, which detects volatile stimuli, and an accessory olfactory system, which detects fluid-phase stimuli. Behavioral evidence suggests that these fluid-phase stimuli often function as pheromones, although pheromones can also be detected by the main olfactory system. In the accessory olfactory system, stimuli are detected by the vomeronasal organ, located in the vomer, between the nose and the mouth. Snakes use it to smell prey, sticking their tongue out and touching it to the organ. Some mammals make a facial expression called flehmen to direct stimuli to this organ.
The sensory receptors of the accessory olfactory system are located in the vomeronasal organ. As in the main olfactory system, the axons of these sensory neurons project from the vomeronasal organ to the accessory olfactory bulb, which in the mouse is located on the dorsal-posterior portion of the main olfactory bulb. Unlike in the main olfactory system, the axons that leave the accessory olfactory bulb do not project to the brain's cortex but rather to targets in the amygdala and bed nucleus of the stria terminalis, and from there to the hypothalamus, where they may influence aggression and mating behavior.
Incest avoidance
The MHC genes (known as HLA in humans) are a group of genes present in many animals and important for the immune system; in general, offspring from parents with differing MHC genes have a stronger immune system. Fish, mice, and female humans are able to smell some aspect of the MHC genes of potential sex partners and prefer partners with MHC genes different from their own.[29][30]
Humans can detect blood relatives from olfaction.[31] Mothers can identify by body odor their biological children but not their stepchildren. Pre-adolescent children can olfactorily detect their full siblings but not half-siblings or step siblings, and this might explain incest avoidance and the Westermarck effect.[32] Functional imaging shows that this olfactory kinship detection process involves the frontal-temporal junction, the insula, and the dorsomedial prefrontal cortex, but not the primary or secondary olfactory cortices, or the related piriform cortex or orbitofrontal cortex.[33]
Olfactory coding and perception
The process by which olfactory information is coded in the brain to allow for proper perception is still being researched, and is not completely understood. When an odorant is detected by receptors, they in a sense break the odorant down, and then the brain puts the odorant back together for identification and perception.[34] The odorant binds to receptors that recognize only a specific functional group, or feature, of the odorant, which is why the chemical nature of the odorant is important.[35]
After binding the odorant, the receptor is activated and will send a signal to the glomeruli.[35] Each glomerulus receives signals from multiple receptors that detect similar odorant features. Because several receptor types are activated due to the different chemical features of the odorant, several glomeruli are activated as well. All of the signals from the glomeruli are then sent to the brain, where the combination of glomeruli activation encodes the different chemical features of the odorant. The brain then essentially puts the pieces of the activation pattern back together in order to identify and perceive the odorant.[35] This distributed code allows the brain to detect specific odors in mixtures of many background odors.[36]
It is a general idea that the layout of brain structures corresponds to physical features of stimuli (called topographic coding), and similar analogies have been made in olfaction with concepts such as a layout corresponding to chemical features (called chemotopy) or perceptual features.[37] While chemotopy remains a highly controversial concept,[38] evidence exists for perceptual information implemented in the spatial dimensions of olfactory networks.[37]
Although conventional wisdom and lay literature, based on impressionistic findings in the 1920s, have long presented human olfaction as capable of distinguishing between roughly 10,000 unique odors, recent research has suggested that the average individual is capable of distinguishing over one trillion unique odors.[39] Researchers in the most recent study, which tested the psychophysical responses to combinations of over 128 unique odor molecules with combinations composed of up to 30 different component molecules, noted that this estimate is "conservative" and that some subjects of their research might be capable of deciphering between a thousand trillion odorants, adding that their worst performer could probably still distinguish between 80 million scents.[40] Authors of the study concluded, "This is far more than previous estimates of distinguishable olfactory stimuli. It demonstrates that the human olfactory system, with its hundreds of different olfactory receptors, far out performs the other senses in the number of physically different stimuli it can discriminate."[41] However, it was also noted by the authors that the ability to distinguish between smells is not analogous to being able to consistently identify them, and that subjects were not typically capable of identifying individual odor stimulants from within the odors the researchers had prepared from multiple odor molecules. In November 2014 the study was strongly criticized by Caltech scientist Markus Meister, who wrote that the study's "extravagant claims are based on errors of mathematical logic".[42][43] The logic of his paper has in turn been criticized by the authors of the original paper.[44]
Genetics
Different people smell different odors, and most of these differences are caused by genetic differences.[45] Although odorant receptor genes make up one of the largest gene families in the human genome, only a handful of genes have been linked conclusively to particular smells. For instance, the odorant receptor OR5A1 and its genetic variants (alleles) are responsible for our ability (or failure) to smell β-ionone, a key aroma in foods and beverages.[46] Similarly, the odorant receptor OR2J3 is associated with the ability to detect the "grassy" odor, cis-3-hexen-1-ol.[47] The preference (or dislike) of cilantro (coriander) has been linked to the olfactory receptor OR6A2.[48]
Interactions with other senses
Olfaction and flavor
Flavor perception is an aggregation of auditory, taste, haptic, and smell sensory information.[1] Retronasal smell plays the biggest role in the sensation of flavor. During the process of mastication, the tongue manipulates food to release odorants. These odorants enter the nasal cavity during exhalation.[49] The olfaction of food has the sensation of being in the mouth because of co-activation of the motor cortex and olfactory epithelium during mastication.[1]
Olfaction, taste, and trigeminal receptors (also called chemesthesis) together contribute to flavor. The human tongue can distinguish only among five distinct qualities of taste, while the nose can distinguish among hundreds of substances, even in minute quantities. It is during exhalation that the olfaction contribution to flavor occurs, in contrast to that of proper smell, which occurs during the inhalation phase of breathing.[49] The olfactory system is the only human sense that bypasses the thalamus and connects directly to the forebrain.[50]
Audition
Olfaction and sound information has been shown to converge in the olfactory tubercles of rodents.[51] This neural convergence is proposed to give rise to a perception termed smound.[52] Whereas a flavor results from interactions between smell and taste, a smound may result from interactions between smell and sound.
Disorders
The following are disorders associated with olfaction:
- Anosmia – inability to smell
- Hyperosmia – an abnormally acute sense of smell
- Hyposmia – decreased ability to smell
- Presbyosmia – the natural decline in the sense of smell in old age[53]
- Dysosmia – distortion in the sense of smell
- Parosmia – distortion in the perception of an odor
- Phantosmia – distortion in the absence of an odor, "hallucinated smell"
- Heterosmia – inability to distinguish odors[53]
- Olfactory reference syndrome – psychological disorder that causes the patient to imagine he or she has strong body odor
- Osmophobia – aversion or psychological hypersensitivity to odors
Quantification in industry
Scientists have devised methods for quantifying the intensity of odors, in particular for the purpose of analyzing unpleasant or objectionable odors released by an industrial source into a community. Since the 1800s industrial countries have encountered incidents where proximity of an industrial source or landfill produced adverse reactions among nearby residents regarding airborne odor. The basic theory of odor analysis is to measure what extent of dilution with "pure" air is required before the sample in question is rendered indistinguishable from the "pure" or reference standard. Since each person perceives odor differently, an "odor panel" composed of several different people is assembled, each sniffing the same sample of diluted specimen air. A field olfactometer can be utilized to determine the magnitude of an odor.
Many air management districts in the US have numerical standards of acceptability for the intensity of odor that is allowed to cross into a residential property. For example, the Bay Area Air Quality Management District has applied its standard in regulating numerous industries, landfills, and sewage treatment plants. Example applications this district has engaged are the San Mateo, California, wastewater treatment plant; the Shoreline Amphitheatre in Mountain View, California; and the IT Corporation waste ponds, Martinez, California.
In plants and animals
The tendrils of plants are especially sensitive to airborne volatile organic compounds. Parasites such as dodder make use of this in locating their preferred hosts and locking on to them.[54] The emission of volatile compounds is detected when foliage is browsed by animals. Threatened plants are then able to take defensive chemical measures, such as moving tannin compounds to their foliage. (See Plant perception).
The importance and sensitivity of smell varies among different organisms; most mammals have a good sense of smell, whereas most birds do not, except the tubenoses (e.g., petrels and albatrosses), certain species of vultures, and the kiwis. Although, recent analysis of the chemical composition of volatile organic compounds (VOCs) from King Penguin feathers suggest that VOCs may provide olfactory cues, used by the penguins to locate their colony and recognise individuals.[55] Among mammals, it is well developed in the carnivores and ungulates, which must always be aware of each other, and in those that smell for their food, such as moles. Having a strong sense of smell is referred to as macrosmatic.
Figures suggesting greater or lesser sensitivity in various species reflect experimental findings from the reactions of animals exposed to aromas in known extreme dilutions. These are, therefore, based on perceptions by these animals, rather than mere nasal function. That is, the brain's smell-recognizing centers must react to the stimulus detected for the animal to be said to show a response to the smell in question. It is estimated that dogs in general have an olfactory sense approximately ten thousand to a hundred thousand times more acute than a human's.[56] This does not mean they are overwhelmed by smells our noses can detect; rather, it means they can discern a molecular presence when it is in much greater dilution in the carrier, air.
Scenthounds as a group can smell one- to ten-million times more acutely than a human, and bloodhounds, which have the keenest sense of smell of any dogs, have noses ten- to one-hundred-million times more sensitive than a human's. They were bred for the specific purpose of tracking humans, and can detect a scent trail a few days old. The second-most-sensitive nose is possessed by the Basset Hound, which was bred to track and hunt rabbits and other small animals.
Bears, such as the Silvertip Grizzly found in parts of North America, have a sense of smell seven times stronger than that of the bloodhound, essential for locating food underground. Using their elongated claws, bears dig deep trenches in search of burrowing animals and nests as well as roots, bulbs, and insects. Bears can detect the scent of food from up to eighteen miles away; because of their immense size, they often scavenge new kills, driving away the predators (including packs of wolves and human hunters) in the process.
The sense of smell is less developed in the catarrhine primates, and nonexistent in cetaceans, which compensate with a well-developed sense of taste. In some strepsirrhines, such as the red-bellied lemur, scent glands occur atop the head. In many species, olfaction is highly tuned to pheromones; a male silkworm moth, for example, can sense a single molecule of bombykol.
Fish, too, have a well-developed sense of smell, even though they inhabit an aquatic environment. Salmon utilize their sense of smell to identify and return to their home stream waters. Catfish use their sense of smell to identify other individual catfish and to maintain a social hierarchy. Many fishes use the sense of smell to identify mating partners or to alert to the presence of food.
Insect olfactory system
Inbreeding avoidance
Since inbreeding is detrimental, it tends to be avoided. In the house mouse, the major urinary protein (MUP) gene cluster provides a highly polymorphic scent signal of genetic identity that appears to underlie kin recognition and inbreeding avoidance. Thus, there are fewer matings between mice sharing MUP haplotypes than would be expected if there were random mating.[57]
See also
- Chemesthesis
- Electronic nose
- Machine olfaction
- Nasal administration olfactory transfer
- Odor
- Olfactometer
- Olfactory ensheathing glia
- Olfactory fatigue
- Scent transfer unit
- Sniffing (behavior)
- Vibration Theory of Olfaction
- Evolution of olfaction
References
- 1933-, Shepherd, Gordon M. (16 July 2013). Neurogastronomy : how the brain creates flavor and why it matters. ISBN 9780231159111. OCLC 882238865.
- de March, Claire A.; Ryu, SangEun; Sicard, Gilles; Moon, Cheil; Golebiowski, Jérôme (September 2015). "Structure–odour relationships reviewed in the postgenomic era". Flavour and Fragrance Journal. 30 (5): 342–361. doi:10.1002/ffj.3249.
- Schacter, Daniel; Gilbert, Daniel; Wegner, Daniel (2011). "Sensation and Perception". Psychology. Worth Publishers. pp. 166–171. ISBN 978-1-4292-3719-2.
- Boroditsky, Lera (1999). "Taste, Smell, and Touch: Lecture Notes" (PDF). p. 1.
- "Olfactory Impairment & Traumatic Brain Injury in blast-injured Combat Troops".
- "Detection of Neurodegerative Disease using Olfaction".
- G. A. Kimble; K. Schlesinger (1985). Topics in the History of Psychology, Volume 1. L. Erlbaum Associates.
- Buck L, Axel R (April 1991). "A novel multigene family may encode odorant receptors: a molecular basis for odor recognition". Cell. 65 (1): 175–87. doi:10.1016/0092-8674(91)90418-X. PMID 1840504.
- Gilad Y, Man O, Pääbo S, Lancet D (March 2003). "Human specific loss of olfactory receptor genes". PNAS. 100 (6): 3324–7. Bibcode:2003PNAS..100.3324G. doi:10.1073/pnas.0535697100. PMC 152291. PMID 12612342.
- Pinel, John P.J. (2006) Biopsychology. Pearson Education Inc. ISBN 0-205-42651-4 (page 178)
- Saberi M, Seyed-allaei (2016). "Odorant receptors of Drosophila are sensitive to the molecular volume of odorants". Scientific Reports. 6: 25103. Bibcode:2016NatSR...625103S. doi:10.1038/srep25103. PMC 4844992. PMID 27112241.
- Turin L (December 1996). "A spectroscopic mechanism for primary olfactory reception". Chemical Senses. 21 (6): 773–91. doi:10.1093/chemse/21.6.773. PMID 8985605.
- Turin L (June 2002). "A method for the calculation of odor character from molecular structure". Journal of Theoretical Biology. 216 (3): 367–85. doi:10.1006/jtbi.2001.2504. PMID 12183125.
- Keller A, Vosshall LB (April 2004). "A psychophysical test of the vibration theory of olfaction". Nature Neuroscience. 7 (4): 337–8. doi:10.1038/nn1215. PMID 15034588. See also the editorial on p. 315.
- Doty, R. L. (2001). Olfaction. 425.
- Bear, Connors and Paradiso, Mark, Barry and Michael (2007). Neuroscience: Exploring the Brain. USA: Lippincott Williams & Wilkins. pp. 265–275.
- Liberles, Stephen (2006). "A second class of chemosensory receptors in the olfactory epithelium". Nature. 442 (7103): 645–650. doi:10.1038/nature05066. PMID 16878137.
- Fulle, HJ (1995). "A receptor guanylyl cyclase expressed specifically in olfactory sensory neurons". Proc Natl Acad Sci USA. 92 (8): 3571–3575. doi:10.1073/pnas.92.8.3571. PMC 42209. PMID 7724600.
- Omura, M (2015). "Trpc2-expressing sensory neurons in the mouse main olfactory epithelium of type B express the soluble guanylate cyclase Gucy1b2". Mol Cell Neurosci. 65: 114–124. doi:10.1016/j.mcn.2015.02.012. PMC 4396857. PMID 25701815.
- Lenders-Zufall, Trese (2007). "Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium". Proc Natl Acad Sci USA. 104 (36): 14507–14512. doi:10.1073/pnas.0704965104. PMC 1964822. PMID 17724338.
- Munger, Steven (2010). "An olfactory subsystem that detects carbon disulfide and mediates food-related social learning". Curr Biol. 20 (16): 1438–1444. doi:10.1016/j.cub.2010.06.021. PMC 2929674. PMID 20637621.
- Bleymehl, K (2016). "A Sensor for Low Environmental Oxygen in the Mouse Main Olfactory Epithelium". Neuron. 92 (6): 1196–1203. doi:10.1016/j.neuron.2016.11.001. PMC 5196021. PMID 27916458.
- Morris, H., & Schaeffer, J. P. (1953). The Nervous system-The Brain or Encephalon. Human anatomy; a complete systematic treatise. (11th ed., pp.1218–1219). New York: Blakiston.
- Rothermel Markus; et al. (2014). "Cholinergic Inputs from Basal Forebrain Add an Excitatory Bias to Odor Coding in the Olfactory Bulb". J. Neurosci. 34 (13): 4654–4664. doi:10.1523/JNEUROSCI.5026-13.2014. PMC 3965788. PMID 24672011.
- Kapoor Vikrant; et al. (2016). "Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels". Nat. Neurosci. 19 (2): 813–4. doi:10.1038/nn.4219. PMC 4948943. PMID 26752161.
- Shea Stephen D.; et al. (2008). "Noradrenergic Induction of Odor-Specific Neural Habituation and Olfactory Memories". J. Neurosci. 28 (42): 10711–10719. doi:10.1523/JNEUROSCI.3853-08.2008. PMC 2588668. PMID 18923046.
- Margot C (July 2009). "A noseful of objects". Nat. Neurosci. 12 (7): 813–4. doi:10.1038/nn0709-813. PMID 19554043.
- Zhou W, Chen D (2009). "Binaral rivalry between the nostrils and in the cortex". Curr Biol. 19 (18): 1561–5. doi:10.1016/j.cub.2009.07.052. PMC 2901510. PMID 19699095.
- Boehm T, Zufall F (February 2006). "MHC peptides and the sensory evaluation of genotype". Trends in Neurosciences. 29 (2): 100–7. doi:10.1016/j.tins.2005.11.006. PMID 16337283.
- Santos PS, Schinemann JA, Gabardo J, Bicalho Mda G (April 2005). "New evidence that the MHC influences odor perception in humans: a study with 58 Southern Brazilian students". Hormones and Behavior. 47 (4): 384–8. doi:10.1016/j.yhbeh.2004.11.005. PMID 15777804.
- Porter RH, Cernoch JM, Balogh RD (1985). "Odor signatures and kin recognition". Physiol Behav. 34 (3): 445–8. doi:10.1016/0031-9384(85)90210-0. PMID 4011726.
- Weisfeld GE, Czilli T, Phillips KA, Gall JA, Lichtman CM (July 2003). "Possible olfaction-based mechanisms in human kin recognition and inbreeding avoidance". Journal of Experimental Child Psychology. 85 (3): 279–95. doi:10.1016/S0022-0965(03)00061-4. PMID 12810039.
- Lundström JN, Boyle JA, Zatorre RJ, Jones-Gotman M (August 2009). "The neuronal substrates of human olfactory based kin recognition". Human Brain Mapping. 30 (8): 2571–80. doi:10.1002/hbm.20686. PMID 19067327.
- Wilson DA (June 2001). "Receptive fields in the rat piriform cortex". Chemical Senses. 26 (5): 577–84. doi:10.1093/chemse/26.5.577. PMID 11418503.
- Leon M, Johnson BA (2003). "Olfactory coding in the mammalian olfactory bulb". Brain Res. Brain Res. Rev. 42 (1): 23–32. doi:10.1016/S0165-0173(03)00142-5. PMID 12668289.
- Rokni, D.; et al. (2014). "An olfactory cocktail party: figure-ground segregation of odorants in rodents" (PDF). Nature Neuroscience. 17 (9): 1225–1232. doi:10.1038/nn.3775. PMC 4146660. PMID 25086608.
- Auffarth, B. (2013). "Understanding smell -- the olfactory stimulus problem". Neuroscience & Biobehavioral Reviews. 37 (8): 1667–1679. doi:10.1016/j.neubiorev.2013.06.009. PMID 23806440.
- Soucy, Edward R.; et al. (2009). "Precision and diversity in an odor map on the olfactory bulb" (PDF). Nature Neuroscience. 12 (2): 210–220. doi:10.1038/nn.2262. PMID 19151709.
- Helen Briggs (20 March 2014). "Nose can detect one trillion odours". BBC News. pp. 23 March 2014.
- Sarah C.P. Williams (20 March 2014). "Human Nose Can Detect a Trillion Smells". Science Now/AAAS News. Retrieved 23 March 2014.
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. (21 March 2014). "Humans Can Discriminate More than 1 million Olfactory Stimuli". Science. 343 (6177): 1370–1372. Bibcode:2014Sci...343.1370B. doi:10.1126/science.1249168. PMC 4483192. PMID 24653035.
- Meister, M. (2014). "Can Humans Really Discriminate 1 Trillion Odors?". eLife. 4: e07865. arXiv:1411.0165. doi:10.7554/eLife.07865. PMC 4491593. PMID 26151672.
- Meister, M. (2015). "On the dimensionality of odor space". eLife. 4: e07865. doi:10.7554/eLife.07865. PMC 4491593. PMID 26151672.
- Magnasco, M.O.; et al. (2015). "On the dimensionality of olfactory space". bioRxiv 022103.
- Howgego, J. (2013) Sense for scents traced down to genes. Nature News, 1 Aug 2013
- Jaeger SR, McRae JF, Bava CM, Beresford MK, Hunter D, Jia Y, Chheang SL, Jin D, Peng M, Gamble JC, Atkinson KR, Axten LG, Paisley AG, Tooman L, Pineau B, Rouse SA, Newcomb RD (2013). "A Mendelian Trait for Olfactory Sensitivity Affects Odor Experience and Food Selection". Current Biology. 23 (16): 1601–1605. doi:10.1016/j.cub.2013.07.030. PMID 23910657.
- McRae JF, Mainland JD, Jaeger SR, Adipietro KA, Matsunami H, Newcomb RD (2012). "Genetic Variation in the Odorant Receptor OR2J3 is Associated with the Ability to Detect the "Grassy" Smelling Odor, cis-3-hexen-1-ol". Chemical Senses. 37 (7): 585–593. doi:10.1093/chemse/bjs049. PMC 3408771. PMID 22714804.
- Callaway, E. (2012) Soapy taste of coriander linked to genetic variants Nature News, 12 Sep 2012
- Masaoka Y, Satoh H, Akai L, Homma I (2010). "Expiration: The moment we experience retronasal olfaction in flavor". Neurosci. Lett. 473 (2): 92–96. doi:10.1016/j.neulet.2010.02.024. PMID 20171264.
- Annual Review of Psychology (February 2001), 52 (1), pg. 423–452, Richard L. Doty
- Wesson DW, Wilson DA (2010). "Smelling Sounds: Olfactory-auditory convergence in the olfactory tubercle". J Neurosci. 30 (8): 3013–3021. doi:10.1523/JNEUROSCI.6003-09.2010. PMC 2846283. PMID 20181598.
- Peeples, Lynne. "Scientific American, Making scents of sounds Lynne Peeples, 23 February 2010, accessed 25 February 2010". Scientificamerican.com. Retrieved 30 December 2012.
- Hawkes, Christopher H. (2002). Smell and taste complaints. Boston: Butterworth-Heinemann. pp. 49–50. ISBN 978-0-7506-7287-0.
- Fountain, Henry (3 October 2006). "This Plant Has the Sense of Smell (Loves Tomatoes, Hates Wheat)". The New York Times.
- Gabirot, M.; Buatois, B.; Müller, T. C.; Bonadonna, F. (2018). "Odour of King Penguin feathers analysed using direct thermal desorption discriminates between individuals but not sexes" (PDF). Ibis. 160 (2): 379–389. doi:10.1111/ibi.12544.
- Peter Tyson (4 October 2012). "Dogs' Dazzling Sense of Smell: What lies behind their exceptional gift of sniff?". PBS. Retrieved 2 November 2016.
- Sherborne AL, Thom MD, Paterson S, Jury F, Ollier WE, Stockley P, Beynon RJ, Hurst JL (2007). "The genetic basis of inbreeding avoidance in house mice". Curr. Biol. 17 (23): 2061–6. doi:10.1016/j.cub.2007.10.041. PMC 2148465. PMID 17997307.
Further reading
- Gordon M.s Shepherd Neurogastronomy: How the Brain Creates Flavor and Why It Matters New York : Columbia University Press, 2012 ISBN 978-0-231-15910-4
External links
| Wikimedia Commons has media related to Olfactory system. |
- Keller A, Vosshall LB (2008). "Better smelling through genetics: mammalian odor perception". Curr. Opin. Neurobiol. 18 (4): 364–9. doi:10.1016/j.conb.2008.09.020. PMC 2590501. PMID 18938244.
- Research on Interesting Questions About Smells
- Insect Olfaction of Plant Odour
- Smells and Odours - How Smell Works at thenakedscientists.com
- Olfaction at cf.ac.uk
- Structure-odor relations: a modern perspective at flexitral.com (PDF)
- Chirality & Odour Perception at leffingwell.com
- ScienceDaily Artille 08/03/2006, Quick -- What's That Smell? Time Needed To Identify Odors Reveals Much About Olfaction at sciencedaily.com
- Scents and Emotions Linked by Learning, Brown Study Shows at brown.edu.com
- Sense of Smell Institute at senseofsmell.org. Research arm of international fragrance industry's The Fragrance Foundation
- Olfactory Systems Laboratory at Boston University
- Smells Database
- Olfaction and Gustation, Neuroscience Online (electronic neuroscience textbook by UT Houston Medical School)
- Digital Olfaction Society, - 3rd World Congress on Digital Olfaction