Quisinostat
Quisinostat (USAN;[2] development code JNJ-26481585) is an experimental drug candidate for the treatment of cancer. It is a "second generation" histone deacetylase inhibitor with antineoplastic activity.[3][4][5] It is highly potent against class I and II HDACs.[6]
 | |
| Clinical data | |
|---|---|
| Other names | JNJ-26481585 |
| ATC code |
|
| Pharmacokinetic data | |
| Bioavailability | oral[1] |
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
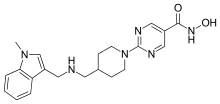
| Formula | C21H26N6O2 |
| Molar mass | 394.470 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
It was developed by Janssen Pharmaceuticals and licensed to NewVac LLC.[7]
Preclinical studies show that quisinostat amplifies HDAC-repressed expression of E-cadherin, leading to a reversal of epithelial to mesenchymal transition in tumor cells.[7] Results of a phase I trials in patients with multiple myeloma in combination with bortezomib and dexamethasone were published in 2016.[8]
References
- "NCI Drug Dictionary". National Cancer Institute. 2 February 2011.
- "Quisinostat" (PDF). American Medical Association.
- Tong, Wei-Gang; Wei, Yue; Stevenson, William; Kuang, Shao-Qing; Fang, Zhihong; Zhang, Ming; Arts, Janine; Garcia-Manero, Guillermo (2010). "Preclinical antileukemia activity of JNJ-26481585, a potent second-generation histone deacetylase inhibitor". Leukemia Research. 34 (2): 221–8. doi:10.1016/j.leukres.2009.07.024. PMID 19682743.
- Stühmer, Thorsten; Arts, Janine; Chatterjee, Manik; Borawski, Johanna; Wolff, André; King, Peter; Einsele, Hermann; Leo, Eugen; Bargou, Ralf C. (2010). "Preclinical anti-myeloma activity of the novel HDAC-inhibitor JNJ-26481585". British Journal of Haematology. 149 (4): 529–36. doi:10.1111/j.1365-2141.2010.08126.x. PMID 20331455.
- "Quisinostat". NCI Drug Dictionary. National Cancer Institute.
- Carol, Hernan; Gorlick, Richard; Kolb, E. Anders; Morton, Christopher L.; Manesh, Donya Moradi; Keir, Stephen T.; Reynolds, C. Patrick; Kang, Min H.; Maris, John M.; Wozniak, Amy; Hickson, Ian; Lyalin, Dmitry; Kurmasheva, Raushan T.; Houghton, Peter J.; Smith, Malcolm A.; Lock, Richard (2014). "Initial testing (stage 1) of the histone deacetylase inhibitor, quisinostat (JNJ-26481585), by the Pediatric Preclinical Testing Program". Pediatric Blood & Cancer. 61 (2): 245–52. doi:10.1002/pbc.24724. PMC 4225045. PMID 24038993.
- LLC, NewVac. "NewVac Reports Primary Endpoint Met in Phase II Clinical Trial of Quisinostat in Combination with Paclitaxel and Carboplatin in Platinum-Resistant Ovarian Cancer". www.prnewswire.com.
- Moreau, Philippe; Facon, Thierry; Touzeau, Cyrille; Benboubker, Lotfi; Delain, Martine; Badamo-Dotzis, Julie; Phelps, Charles; Doty, Christopher; Smit, Hans; Fourneau, Nele; Forslund, Ann; Hellemans, Peter; Leleu, Xavier (2016). "Quisinostat, bortezomib, and dexamethasone combination therapy for relapsed multiple myeloma". Leukemia & Lymphoma. 57 (7): 1546–59. doi:10.3109/10428194.2015.1117611. PMID 26758913.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.