Givinostat
Givinostat (INN[1]) or gavinostat (originally ITF2357) is a histone deacetylase inhibitor with potential anti-inflammatory, anti-angiogenic, and antineoplastic activities.[2] It is a hydroxamate used in the form of its hydrochloride.
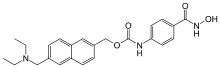 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.258.524 |
| Chemical and physical data | |
| Formula | C24H27N3O4 |
| Molar mass | 421.489 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Givinostat is in numerous phase II clinical trials (including for relapsed leukemias and myelomas),[3] and has been granted orphan drug designation in the European Union for the treatment of systemic juvenile idiopathic arthritis,[4] polycythaemia vera.[5] and Duchenne muscular dystrophy.
A preclinical study produced early results suggesting the molecule might help with diastolic dysfunction.[6]
ITF2357 was discovered at Italfarmaco of Milan, Italy. It was patented in 1997 and first described in the scientific literature in 2005.[7][8]
Adverse effects
In clinical trials of givinostat as a salvage therapy for advanced Hodgkin's lymphoma, the most common adverse reactions were fatigue (seen in 50% of participants), mild diarrhea or abdominal pain (40% of participants), moderate thrombocytopenia (decreased platelet counts, seen in one third of patients), and mild leukopenia (a decrease in white blood cell levels, seen in 30% of patients). One-fifth of patients experienced prolongation of the QT interval, a measure of electrical conduction in the heart, severe enough to warrant temporary suspension of treatment.[9]
Mechanism of action
Givinostat inhibits class I and class II histone deacetylases (HDACs) and several pro-inflammatory cytokines. This reduces expression of tumour necrosis factor (TNF), interleukin 1α and β, and interleukin 6.[8]
It also has activity against cells expressing JAK2(V617F), a mutated form of the janus kinase 2 (JAK2) enzyme that is implicated in the pathophysiology of many myeloproliferative diseases, including polycythaemia vera.[10][11] In patients with polycythaemia, the reduction of mutant JAK2 concentrations by givinostat is believed to slow down the abnormal growth of erythrocytes and ameliorate the symptoms of the disease.[5]
References
- "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended INN: List 63" (PDF). WHO Drug Information. 24 (1): 58–9. 2010.
- National Cancer Institute (2010). "Gavinostat". NCI Cancer Dictionary. U.S. National Institutes of Health. Retrieved 2010-09-15.
- "Search results for ITF2357". ClinicalTrials.gov.
- Committee for Orphan Medicinal Products (23 February 2010). "Public summary of opinion on orphan designation: Givinostat for the treatment of systemic-onset juvenile idiopathic arthritis" (PDF). European Medicines Agency. Retrieved 2010-09-15.
- Committee for Orphan Medicinal Products (3 March 2010). "Public summary of opinion on orphan designation: Givinostat for the treatment of polycythaemia vera" (PDF). European Medicines Agency.
- "Potential treatment for diastolic dysfunction in heart failure". ScienceDaily. Retrieved 2018-08-19.
- WO patent application 1997/043251, "Compounds with anti-inflammatory and immunosuppressive activities", published 1997-11-20, assigned to Italfarmaco S.p.A.
- Leoni, F; Fossati, G (2005). "The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo". Molecular Medicine. 11: 1. doi:10.2119/2006-00005.Dinarello. PMC 1449516. PMID 16557334.
- Tan, J; Cang, S; Ma, Y; Petrillo, RL; Liu, D (2010). "Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents". Journal of Hematology & Oncology. 3: 5. doi:10.1186/1756-8722-3-5. PMC 2827364. PMID 20132536.
- Vannucchi, AM; Guglielmelli, P; Pieri, L; Antonioli, E; Bosi, A (2009). "Treatment options for essential thrombocythemia and polycythemia vera". Expert Review of Hematology. 2 (1): 41–55. doi:10.1586/17474086.2.1.41.
- Guerini, V; Barbui, V; Spinelli, O; et al. (April 2008). "The histone deacetylase inhibitor ITF2357 selectively targets cells bearing mutated JAK2(V617F)". Leukemia. 22 (4): 740–7. doi:10.1038/sj.leu.2405049. PMID 18079739.
Further reading
- Job-Deslandre, C (January 2007). "Idiopathic juvenile-onset systemic arthritis". Orphanet. Orphan number: ORPHA85414.
- Amaru Calzada, Ariel; Todoerti, Katia; Donadoni, Luca; Pellicioli, Anna; Tuana, Giacomo; Gatta, Raffaella; Neri, Antonino; Finazzi, Guido; Mantovani, Roberto; Rambaldi, Alessandro; Introna, Martino; Lombardi, Luigia; Golay, Josée (August 2012). "The HDAC inhibitor Givinostat modulates the hematopoietic transcription factors NFE2 and C-MYB in JAK2(V617F) myeloproliferative neoplasm cells". Experimental Hematology. 40: 634–645.e10. doi:10.1016/j.exphem.2012.04.007. PMID 22579713.