Medicinal fungi
Medicinal fungi are those fungi which produce medically significant metabolites or can be induced to produce such metabolites using biotechnology. The range of medically active compounds that have been identified include antibiotics, anti-cancer drugs, cholesterol inhibitors, psychotropic drugs, immunosuppressants and even fungicides. Although initial discoveries centred on simple moulds of the type that cause spoilage of food, later work identified useful compounds across a wide range of fungi.
History
Although fungi products have been used in traditional and folk medicines, probably since pre-history, the ability to identify beneficial properties and then extract the active ingredient started with the discovery of penicillin by Alexander Fleming in 1928. Since that time, many additional antibiotics have been discovered and the potential for fungi to synthesize biologically active molecules, useful in a wide range of clinical therapies, has been extensively exploited.
Pharmacological research has now isolated antifungal, antiviral, and antiprotozoan, isolates from fungi.[1]
The fungus with probably the longest record of medicinal use, Ganoderma lucidum, is known in Chinese as líng zhī ("spirit plant"), and in Japanese as mannentake ("10,000-year mushroom"). In ancient Japan, Grifola frondosa was worth its weight in silver, although no significant therapeutic benefits have been demonstrated in humans.[2]
Studies have shown another species of genus Ganoderma, G. applanatum, contains compounds with anti-tumor and anti-fibrotic properties.
Inonotus obliquus was used in Russia as early as the 16th century, and it featured in Alexandr Solzhenitsyn's 1967 novel Cancer Ward.[3]
Applications
Cancer
Paclitaxel is synthesised using Penicillium raistrickii and plant cell fermentation. Fungi can synthesize other mitotic inhibitors including vinblastine, vincristine, podophyllotoxin, griseofulvin, aurantiamine, oxaline, and neoxaline.[6][7]
11,11'-Dideoxyverticillin A, an isolate of marine Penicillium, was used to create dozens of semi-synthetic anticancer compounds.[8] 11,11'-Dideoxyverticillin A, andrastin A, barceloneic acid A, and barceloneic acid B, are farnesyl transferase inhibitors that can be made by Penicillium.[9] 3-O-Methylfunicone, anicequol, duclauxin, and rubratoxin B, are anticancer/cytotoxic metabolites of Penicillium.
Penicillium is a potential source of the leukemia medicine asparaginase.[10]
Some countries have approved Beta-glucan fungal extracts lentinan, polysaccharide-K, and polysaccharide peptide as immunologic adjuvants.[11] Evidence suggests this use as effective in prolonging and improving the quality of life for patients with certain cancers, although the Memorial Sloan-Kettering Cancer Center observes that "well designed, large scale studies are needed to establish the role of lentinan as a useful adjunct to cancer treatment".[12] According to Cancer Research UK, "there is currently no evidence that any type of mushroom or mushroom extract can prevent or cure cancer".[13] Fungal metabolites such as ergosterol, clavilactones, and triterpenoids are efficient Cdk inhibitors that lead to G1/S or G2/M arrest of cancer cells. Other metabolites, such as panepoxydone, are inhibitors of NF-κB. Fucose and mannose fragments of fungal cell wall are antagonists of VEGF-receptors [14]
Antibacterial agents (antibiotics)
Alexander Fleming led the way to the beta-lactam antibiotics with the Penicillium mold and penicillin. Subsequent discoveries included alamethicin, aphidicolin, brefeldin A, Cephalosporin, cerulenin, citromycin, eupenifeldin, fumagillin, fusafungine, fusidic acid, itaconic acid, MT81, nigrosporin B, usnic acid, verrucarin A, vermiculine and many others.
Antibiotics retapamulin, tiamulin, and valnemulin are derivatives of the fungal metabolite pleuromutilin. Plectasin, austrocortilutein, austrocortirubin, coprinol, oudemansin A, strobilurin, illudin, pterulone, and sparassol are antibiotics isolated from basidiomycete species.
Cholesterol biosynthesis inhibitors

Statins are an important class of cholesterol-lowering drugs; the first generation of statins were derived from fungi.[15] Lovastatin, the first commercial statin, was extracted from a fermentation broth of Aspergillus terreus.[15] Industrial production is now capable of producing 70 mg lovastatin per kilogram of substrate.[16] The red yeast rice fungus, Monascus purpureus, can synthesize lovastatin, mevastatin, and the simvastatin precursor monacolin J. Nicotinamide riboside, a cholesterol biosynthesis inhibitor, is made by Saccharomyces cerevisiae.
Antifungals
Some antifungals are derived or extracted from other fungal species. Griseofulvin is derived from a number of Penicillium species, caspofungin is derived from Glarea lozoyensis.[17] Strobilurin, azoxystrobin, micafungin, and echinocandins, are all extracted from fungi. Anidulafungin is a derivative of an Aspergillus metabolite.
Antivirals
Many compounds isolated from the edible and poisonous mushroom has shown a broad spectrum antiviral activity against virus such as HIV, herpes virus, influenza virus, Epstein-Barr virus, coxsackievirus, etc. Even though many studies have proved their activity, but still availability of such compounds as the antiviral drugs is still underdeveloped. Mushrooms such as Lentinus edodes, Ganoderma lucidum, Ganoderma colossus, Hypsizygus marmoreus, Cordyceps militaris, Grifola frondosa, Scleroderma citrinum, Etc. has been shown to contain antiviral compounds.[18][19][20]
Immunosuppressants
Ciclosporin, was discovered in Tolypocladium inflatum. Bredinin was discovered in Eupenicillium brefeldianum. Mycophenolic acid was discovered in Penicillium stoloniferum. Thermophilic fungi were the source of the fingolimod precursor myriocin. Aspergillus synthesizes immunosuppressants gliotoxin and endocrocin. Subglutinols are immunosuppressants isolated from Fusarium subglutinans.[21] Other compounds include mizoribine.
Malaria
Codinaeopsin, efrapeptins, zervamicins, and antiamoebin,[22] are made by fungi.
Diabetes
Many fungal isolates act as DPP-4 inhibitors, alpha-glucosidase inhibitors, and alpha amylase inhibitors in vitro. Ternatin is a fungal isolate that suppresses hyperglycemia.[23] Aspergillusol A is an alpha-glucosidase inhibitor made by Aspergillus. Sclerotiorin is an aldose reductase inhibitor made by Penicillium.
Psychotropic effects
A number of fungi have well documented psychotropic effects, some of them severe and associated with sometimes acute and life-threatening side-effects.[24] Well known amongst these is Amanita muscaria, the fly agaric. More widely used informally are a range of fungi collectively known as "magic mushrooms", which contain psilocybin and psilocin.
The history of bread-making is also peppered with references to deadly ergotism caused by ergot, most commonly Claviceps purpurea, a parasite of cereal crops. A number of therapeutically useful drugs have subsequently been extracted from ergot including ergotamine, pergolide and cabergoline.[25]
Psychotropic compounds created from ergot alkaloids also include dihydroergotamine, methysergide, methylergometrine, hydergine, nicergoline, lisuride, bromocriptine, cabergoline, pergolide. Polyozellus multiplex synthesizes prolyl endopeptidase inhibitors polyozellin, thelephoric acid, kynapcins. Neurotrophic fungal isolates include L-theanine, tricholomalides, scabronines, termitomycesphins. Many fungi synthesize the partial, non-selective, serotonin receptor agonist/analog psilocin.
A number of other fungal species, including species of Aspergillus and Penicillium, have been induced to produce ergot alkaloids.
Vitamins
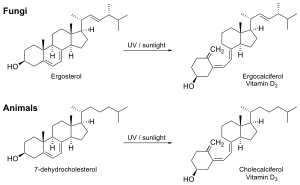
Fungi are a source of ergosterol which can be converted to vitamin D upon exposure to ultraviolet light to synthesize vitamins D2 (ergocalciferol), D4 (22-dihydroergocalciferol), and D1 (Lumisterol+D2).[26][27]
Phytase
Aspergillus niger is used to produce recombinant phytase, an enzyme added to animal feeds to improve absorption of phosphorus.
Edible species containing drugs
Edible species which contain drugs (biologically active constituents) include:
- Agaricus subrufescens (Agaricus blazei/brasiliensis, almond mushroom) is a fungus associated with Brazil and Japan. Blazein, a bioactive steroid, was isolated from A. subrufescens.[28][29]
- The adenosine analog cordycepin was originally isolated from Cordyceps.[30] Other Cordyceps isolates include, cordymin, cordycepsidone, and cordyheptapeptide. CS-4 is commercially sold as C. sinensis, but Cs-4 has recently been confirmed to be a different species from the Cordyceps species used in traditional Chinese medicine. CS-4 is properly known as Paecilomyces hepiali. Hirsutella sinensis is the accepted asexual form of C. sinensis.[31]
- Ganoderma lucidum (Ling zhi, mannentake, reishi) contains p-hydroxybenzoic acid, cinnamic acid, and lanostane-type triterpenoids such as ganoderic acids.[32]
- Hydnellum peckii has yielded atromentin, a compound isolated from the mycorrhiza,[33] and subsequently its biosynthesis has been characterized.[34]
- Lentinula edodes (Shiitake) has been used as a source of Lentinan, AHCC, and eritadenine.[35]
- Schizophyllum commune (Split gill) has yielded schizophyllan (SPG, sizofiran, sonifilan).[36] Hydrophobins were originally isolated from S. commune. A chemically analogous polysaccharide, scleroglucan, is an isolate of Sclerotium rolfsii.
- Tolypocladium inflatum Gams yields the immunosuppressant ciclosporin.[37]
- Trametes versicolor (Coriolus versicolor, yun zhi, kawaratake, turkey tail) have produced protein-bound polysaccharides PSK and PSP (polysaccharopeptide) from different mycelia strains.[38]
- Ustilago maydis (Mexican truffle, huitlacoche, corn fungus) synthesises ustilagine and ustilagic acid.[39]
Yeasts
Saccharomyces is used industrially to produce the amino acid lysine, as well as recombinant proteins insulin and Hepatitis B surface antigen. Transgenic yeast are used to produce artemisinin, as well as a number of insulin analogs.[40] Candida is used industrially to produce vitamins ascorbic acid and riboflavin. Pichia is used to produce the amino acid tryptophan and the vitamin pyridoxine. Rhodotorula is used to produce the amino acid phenylalanine. Moniliella is used industrially to produce the sugar alcohol erythritol.
See also
- Saccharomyces boulardii and Saccharomyces cerevisiae (baker's/brewer's yeast) extracts:
- Vegemite
- Marmite
- Cenovis
- Promite
- Guinness Yeast Extract
- PGG-glucan
- MOS
- Nutritional yeast
- Yeast extract
- Nicotinamide riboside
- Zymosan
References
- Engler M, Anke T, Sterner O (1998). "Production of antibiotics by Collybia nivalis, Omphalotus olearis, a Favolaschia and a Pterula species on natural substrates". Z Naturforsch C. 53 (5–6): 318–24. doi:10.1515/znc-1998-5-604. PMID 9705612.
- "Maitake Mushroom". Complementary and Alternative Medicine : Diet and Nutrition. American Cancer Society. 2008. Retrieved 2011-03-08.
- Zheng, Weifa; Miao, Kangjie; Liu, Yubing; Zhao, Yanxia; Zhang, Meimei; Pan, Shenyuan; Dai, Yucheng (2010). "Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production". Applied Microbiology and Biotechnology. 87 (4): 1237–54. doi:10.1007/s00253-010-2682-4. PMID 20532760.
- Bemani E, Ghanati F, Rezaei A, Jamshidi M (2013). "Effect of phenylalanine on Taxol production and antioxidant activity of extracts of suspension-cultured hazel (Corylus avellana L.) cells". J Nat Med. 67 (3): 446–51. doi:10.1007/s11418-012-0696-1. PMID 22847380.
- Gangadevi V, Murugan M, Muthumary J (2008). "Taxol determination from Pestalotiopsis pauciseta, a fungal endophyte of a medicinal plant". Sheng Wu Gong Cheng Xue Bao. 24 (8): 1433–8. doi:10.1016/s1872-2075(08)60065-5. PMID 18998547.
- Rosaria Nicoletti; Maria Letizia Ciavatta; Elisabetta Buommino; Maria Antonietta Tufano (2008). "Antitumor extrolites produced by Penicillium species" (PDF). International Journal of Biomedical and Pharmaceutical Sciences. 2 (1): 1–23. Archived from the original (PDF) on 26 December 2014. Retrieved 26 August 2016.
- "Antitumor extrolites produced by Penicillium species" (PDF). Archived from the original (PDF) on December 26, 2014. Retrieved August 17, 2014. Cite journal requires
|journal=(help) - "Research update: Chemists find help from nature in fighting cancer - MIT News Office". Web.mit.edu. 2013-02-27. Retrieved 2013-12-17.
- Overy DP, Larsen TO, Dalsgaard PW, Frydenvang K, Phipps R, Munro MH, et al. (2005). "Andrastin A and barceloneic acid metabolites, protein farnesyl transferase inhibitors from Penicillium albocoremium: chemotaxonomic significance and pathological implications". Mycol Res. 109 (Pt 11): 1243–9. doi:10.1017/S0953756205003734. PMID 16279417.
- Shrivastava A, Khan AA, Shrivastav A, Jain SK, Singhal PK (2012). "Kinetic studies of L-asparaginase from Penicillium digitatum". Prep Biochem Biotechnol. 42 (6): 574–81. doi:10.1080/10826068.2012.672943. PMID 23030468.
- Ina, K; Kataoka, T; Ando, T (2013). "The use of lentinan for treating gastric cancer". Anti-Cancer Agents in Medicinal Chemistry. 13 (5): 681–8. doi:10.2174/1871520611313050002. PMC 3664515. PMID 23092289.
- "Lentinan". Memorial Sloan-Kettering Cancer Center. 27 February 2013. Retrieved 26 August 2016.
- "Mushrooms and cancer". Cancer Research UK. 2017-08-30. Retrieved 26 August 2016.
- Zmitrovich IV (2015). "Anti-cancer metabolites of Basidiomycota and their molecular targets. A review" (PDF). Vestnik Permskogo Universiteta. Biologiya. 2015 (3): 264–86.
- Tolbert, Jonathan A. (2003). "Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors". Nature Reviews Drug Discovery. 2 (7): 517–526. doi:10.1038/nrd1112. PMID 12815379.
- Jahromi MF; Liang JB; Ho YW; Mohamad R; Goh YM; Shokryazdan P (2012). "Lovastatin production by Aspergillus terreus using agro-biomass as substrate in solid state fermentation". J Biomed Biotechnol. 2012: 1–11. doi:10.1155/2012/196264. PMC 3478940. PMID 23118499.
- Richardson, Malcolm D.; Warnock, David W. (2003-12-01). Fungal Infection Diagnosis and Management. ISBN 978-1-4051-15780.
- Pradeep, Prabin; Manju, Vidya; Ahsan, Mohammad Feraz (2019), Agrawal, Dinesh Chandra; Dhanasekaran, Muralikrishnan (eds.), "Antiviral Potency of Mushroom Constituents", Medicinal Mushrooms: Recent Progress in Research and Development, Springer Singapore, pp. 275–297, doi:10.1007/978-981-13-6382-5_10, ISBN 9789811363825
- Hassan, Mohamed; Rouf, Razina; Tiralongo, Evelin; May, Tom; Tiralongo, Joe (2015-04-08). "Mushroom Lectins: Specificity, Structure and Bioactivity Relevant to Human Disease". International Journal of Molecular Sciences. 16 (12): 7802–7838. doi:10.3390/ijms16047802. ISSN 1422-0067. PMC 4425051. PMID 25856678.
- Friedman, Mendel (December 2016). "Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans". Foods. 5 (4): 80. doi:10.3390/foods5040080. PMC 5302426. PMID 28231175.
- Kim H, Baker JB, Park Y, Park HB, DeArmond PD, Kim SH, et al. (2010). "Total synthesis, assignment of the absolute stereochemistry, and structure-activity relationship studies of subglutinols A and B.". Chem Asian J. 5 (8): 1902–10. doi:10.1002/asia.201000147. PMID 20564278.
- Nagaraj, G.; Uma, MV.; Shivayogi, MS.; Balaram, H. (January 2001). "Antimalarial activities of peptide antibiotics isolated from fungi". Antimicrobial Agents and Chemotherapy. 45 (1): 145–9. doi:10.1128/aac.45.1.145-149.2001. PMC 90252. PMID 11120957.
- Lo, HC; Wasser, SP (2011). "Medicinal mushrooms for glycemic control in diabetes mellitus: History, current status, future perspectives, and unsolved problems (review)". International Journal of Medicinal Mushrooms. 13 (5): 401–26. doi:10.1615/intjmedmushr.v13.i5.10. PMID 22324407.
- Hoegberg LC; Larsen L; Sonne L; Bang J; Skanning PG (2008). "Three cases of Amanita muscaria ingestion in children: two severe courses [abstract]". Clinical Toxicology. 46 (5): 407–8. doi:10.1080/15563650802071703. PMID 18568796.
- Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E (January 2007). "Dopamine agonists and the risk of cardiac-valve regurgitation". The New England Journal of Medicine. 356 (1): 29–38. doi:10.1056/NEJMoa062222. PMID 17202453.
- Keegan RJ, Lu Z, Bogusz JM, Williams JE, Holick MF (Jan 1, 2013). "Photobiology of vitamin D in mushrooms and its bioavailability in humans". Dermatoendocrinol. 5 (1): 165–76. doi:10.4161/derm.23321. PMC 3897585. PMID 24494050.
- Kamweru, PK; Tindibale, EL (2016). "Vitamin D and Vitamin D from Ultraviolet-Irradiated Mushrooms (Review)". International Journal of Medicinal Mushrooms. 18 (3): 205–14. doi:10.1615/IntJMedMushrooms.v18.i3.30. PMID 27481154.
- Itoh, H; Ito, H; Hibasami, H (December 2008). "Blazein of a new steroid isolated from Agaricus blazei Murrill (himematsutake) induces cell death and morphological change indicative of apoptotic chromatin condensation in human lung cancer LU99 and stomach cancer KATO III cells". Oncology Reports. 20 (6): 1359–61. doi:10.3892/or_00000152. PMID 19020714.
- Wang, H; Fu, Z; Han, C (2013). "The Medicinal Values of Culinary-Medicinal Royal Sun Mushroom (Agaricus blazei Murrill)". Evidence-Based Complementary and Alternative Medicine. 2013: 842619. doi:10.1155/2013/842619. PMC 3833359. PMID 24288568.
- Cunningham, K. G.; Manson, W.; Spring, F. S. & Hutchinson, S. A. (1950). "Cordycepin, a Metabolic Product isolated from Cultures of Cordyceps militaris (Linn.) Link". Nature. 166 (4231): 949. Bibcode:1950Natur.166..949C. doi:10.1038/166949a0. PMID 14796634.
- Chen, Yue-Qin; Wang, Ning; Qu, Liang-Hu; Li, Tai-Hui; Zhang, Wei-Ming (2001). "Determination of the anamorph of Cordyceps sinensis inferred from the analysis of the ribosomal DNA internal transcribed spacers and 5.8S rDNA". Biochemical Systematics and Ecology. 29 (6): 597–607. doi:10.1016/S0305-1978(00)00100-9. ISSN 0305-1978. PMID 11336809.
- Liu D, Gong J, Dai W, Kang X, Huang Z, Zhang HM, et al. (2012). "The genome of Ganoderma lucidum provides insights into triterpenes biosynthesis and wood degradation [corrected]". PLoS ONE. 7 (5): e36146. doi:10.1371/journal.pone.0036146. PMC 3342255. PMID 22567134.
- Khanna, Jatinder M.; Malone, Marvin H.; Euler, Kenneth L.; Brady, Lynn R. (1965). "Atromentin. Anticoagulant from Hydnellum diabolus". Journal of Pharmaceutical Sciences. 54 (7): 1016–20. doi:10.1002/jps.2600540714. PMID 5862512.
- Schneider, P; Bouhired, S; Hoffmeister, D (November 2008). "Characterization of the atromentin biosynthesis genes and enzymes in the homobasidiomycete Tapinella panuoides". Fungal Genetics and Biology. 45 (11): 1487–96. doi:10.1016/j.fgb.2008.08.009. PMID 18805498.
- Bisen, PS; Baghel, RK; Sanodiya, BS; Thakur, GS; Prasad, GB (2010). "Lentinus edodes: a macrofungus with pharmacological activities". Current Medicinal Chemistry. 17 (22): 2419–30. doi:10.2174/092986710791698495. PMID 20491636.
- Mantovani G, Bianchi A, Curreli L, Ghiani M, Astara G, Lampis B, et al. (1997). "Clinical and immunological evaluation of schizophyllan (SPG) in combination with standard chemotherapy in patients with head and neck squamous cell carcinoma". Int J Oncol. 10 (1): 213–21. doi:10.3892/ijo.10.1.213. PMID 21533366.
- Borel JF, Kis ZL, Beveridge T (1995). "The history of the discovery and development of Cyclosporin (Sandimmune®)". In Merluzzi VJ, Adams J (eds.). The search for anti-inflammatory drugs case histories from concept to clinic. Boston: Birkhäuser. pp. 27–63. ISBN 978-1-4615-9846-6. Archived from the original on 2017-11-05.
- PDQ Integrative, Alternative, and Complementary Therapies Editorial, Board (2002). "Medicinal Mushrooms (PDQ®): Patient Version". PDQ Cancer Information Summaries. National Cancer Institute (US). PMID 28267306.CS1 maint: multiple names: authors list (link)
- Juárez-Montiel M, Ruiloba de León S, Chávez-Camarillo G, Hernández-Rodríguez C, Villa-Tanaca L (2011). "Huitlacoche (corn smut), caused by the phytopathogenic fungus Ustilago maydis, as a functional food". Rev Iberoam Micol. 28 (2): 69–73. doi:10.1016/j.riam.2011.01.001. PMID 21352944.
- Mark Peplow. "Sanofi launches malaria drug production | Chemistry World". Rsc.org. Retrieved 2013-12-17.
External links
- Memorial Sloan-Kettering Agaricus subrufescens, Phellinus linteus, Ganoderma lucidum, Trametes versicolor and PSK, Grifola frondosa, Inonotus obliquus, Pleurotus ostreatus, Cordyceps, Shiitake, Lentinan, AHCC.
- American Cancer Society Trametes versicolor and PSK, Grifola frondosa, Shiitake.
- National Cancer Institute Shiitake, Lentinan, Cordycepin