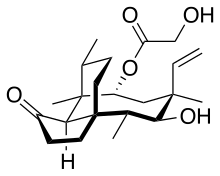
Pleuromutilin
Pleuromutilin and its derivatives are antibacterial drugs that inhibit protein synthesis in bacteria by binding to the peptidyl transferase component of the 50S subunit of ribosomes.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(4R,5S,6S,8R,9aR,10R)-6-Ethenyl-5-hydroxy-4,6,9,10-tetramethyl-1-oxodecahydro-3a,9-propanocyclopenta[8]annulen-8-yl hydroxyacetate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.316 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C22H34O5 |
| Molar mass | 378.509 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
This class of antibiotics includes the licensed drugs lefamulin (for systemic use in humans), retapamulin (approved for topical use in humans), valnemulin and tiamulin (approved for use in animals) and the investigational drug azamulin.
History
Pleuromutilin was discovered as an antibiotic in 1950.[2] It is derived from the fungus Clitopilus passeckerianus (formerly Pleurotus passeckerianus), and has also been found in Drosophila subatrata, Clitopilus scyphoides, and some other Clitopilus species.[3]
Additional reading
- Long, Katherine S; Lykke H. Hansen; Lene Jakobsen; Birte Vester (April 2006). "Interaction of Pleuromutilin Derivatives with the Ribosomal Peptidyl Transferase Center" (PDF). Antimicrobial Agents and Chemotherapy. 50 (4): 1458–1462. doi:10.1128/AAC.50.4.1458-1462.2006. PMC 1426994. PMID 16569865.
- Lolk, L.; Pøhlsgaard, J.; Jepsen, A. S.; Hansen, L. H.; Nielsen, H.; Steffansen, S. I.; Sparving, L.; Nielsen, A. B.; Vester, B.; Nielsen, P. (2008). "A Click Chemistry Approach to Pleuromutilin Conjugates with Nucleosides or Acyclic Nucleoside Derivatives and Their Binding to the Bacterial Ribosome". Journal of Medicinal Chemistry. 51 (16): 4957–4967. doi:10.1021/jm800261u. PMID 18680270.
References
- Sonia Ilaria Maffioli (2014). "A Chemist's Survey of Different Antibiotic Classes". In Claudio O. Gualerzi, Letizia Brandi, Attilio Fabbretti, Cynthia L. Pon. (eds.). Antibiotics: Targets, Mechanisms and Resistance. Wiley-VCH. ISBN 9783527659685.CS1 maint: uses editors parameter (link)
- Novak, R; Shlaes DM (February 2010). "The pleuromutilin antibiotics: a new class for human use". Current Opinion in Investigational Drugs. 11 (2): 182–91. PMID 20112168.
- Kilaru, Sreedhar; Catherine M. Collins; Amanda J. Hartley; Andy M. Bailey; Gary D. Foster (2009-09-18). "Establishing Molecular Tools for Genetic Manipulation of the Pleuromutilin-Producing Fungus Clitopilus passeckerianus". Appl Environ Microbiol. American Society for Microbiology. 75 (22): 7196–7204. doi:10.1128/AEM.01151-09. PMC 2786515. PMID 19767458.
- Total synthesis of (.+-.)-pleuromutilin E. Grant Gibbons Journal of the American Chemical Society 1982 104 (6), 1767-1769 doi: 10.1021/ja00370a067
- Synthetic studies directed toward naturally occurring cyclooctanoids. 2. A stereocontrolled assembly of (.+-.)-pleuromutilin via a remarkable sterically demanding oxy-Cope rearrangement Robert K. Boeckman Jr., Dane M. Springer, and Thomas R. Alessi Journal of the American Chemical Society 1989 111 (21), 8284-8286 doi:10.1021/ja00203a043
- Fazakerley, N. J., Helm, M. D. and Procter, D. J. (2013), Total Synthesis of (+)-Pleuromutilin. Chem. Eur. J., 19: 6718–6723. doi:10.1002/chem.201300968
- A modular and enantioselective synthesis of the pleuromutilin antibiotics Stephen K. Murphy, Mingshuo Zeng, Seth B. Herzon Science 02 Jun 2017: Vol. 356, Issue 6341, pp. 956-959 doi:10.1126/science.aan0003
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.