Hip replacement
Hip replacement is a surgical procedure in which the hip joint is replaced by a prosthetic implant, that is, a hip prosthesis. Hip replacement surgery can be performed as a total replacement or a hemi (half) replacement. Such joint replacement orthopaedic surgery is generally conducted to relieve arthritis pain or in some hip fractures. A total hip replacement (total hip arthroplasty or THA) consists of replacing both the acetabulum and the femoral head while hemiarthroplasty generally only replaces the femoral head. Hip replacement is currently one of the most common orthopaedic operations, though patient satisfaction short- and long-term varies widely. Approximately 58% of total hip replacements are estimated to last 25 years.[1] The average cost of a total hip replacement in 2012 was $40,364 in the United States, and about $7,700 to $12,000 in most European countries.[2]
| Hip replacement | |
|---|---|
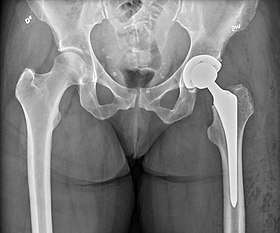 | |
| Other names | Hip athroplasty |
| ICD-9-CM | 81.51–81.53 |
| MeSH | D019644 |
| MedlinePlus | 002975 |
Medical uses
Total hip replacement is most commonly used to treat joint failure caused by osteoarthritis. Other indications include rheumatoid arthritis, avascular necrosis, traumatic arthritis, protrusio acetabuli, certain hip fractures, benign and malignant bone tumors, arthritis associated with Paget's disease, ankylosing spondylitis and juvenile rheumatoid arthritis. The aims of the procedure are pain relief and improvement in hip function. Hip replacement is usually considered only after other therapies, such as physical therapy and pain medications, have recently failed.
Risks
Risks and complications in hip replacement are similar to those associated with all joint replacements. They can include infection, dislocation, limb length inequality, loosening, impingement, osteolysis, metal sensitivity, nerve palsy, chronic pain and death. Weight loss surgery before a hip replacement does not appear to change outcomes.[3]
Infection
Infection is one of the most common causes for revision of a total hip replacement, along with loosening and dislocation. The incidence of infection in primary hip replacement is around 1% or less in the United States.[4] Risk factors for infection include obesity, diabetes, smoking, immunosuppressive medications or diseases, and history of infection.
Modern diagnosis of infection around a total knee replacement is based on the Musculoskeletal Infection Society (MSIS) criteria.[5] They are:
- There is a sinus tract communicating with the prosthesis; or
- A pathogen is isolated by culture from at least two separate tissue or fluid samples obtained from the affected prosthetic joint;
or
Four of the following six criteria exist:
- Elevated serum erythrocyte sedimentation rate (ESR>30mm/hr) and serum C-reactive protein (CRP>10 mg/L) concentration,
- Elevated synovial leukocyte count,
- Elevated synovial neutrophil percentage (PMN%),
- Presence of purulence in the affected joint,
- Isolation of a microorganism in one culture of periprosthetic tissue or fluid, or
- Greater than five neutrophils per high-power field in five high-power fields observed from histologic analysis of periprosthetic tissue at ×400 magnification.
None of the above laboratory tests has 100% sensitivity or specificity for diagnosing infection. Specificity improves when the tests are performed in patients in whom clinical suspicion exists. ESR and CRP remain good 1st line tests for screening (high sensitivity, low specificity). Aspiration of the joint remains the test with the highest specificity for confirming infection.
Dislocation
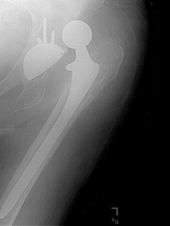
Dislocation is the most common complication of hip replacement surgery. The most common causes vary by the duration since the surgery.
Hip prosthesis dislocation mostly occurs in the first three months after insertion, mainly because of incomplete scar formation and relaxed soft tissues.[6] It takes eight to twelve weeks for the soft tissues injured or cut during surgery to heal. During this period, the hip ball can come out of the socket. The chance of this is diminished if less tissue is cut, if the tissue cut is repaired and if large diameter head balls are used.
Dislocations occurring between three months and five years after insertion usually occur due to malposition of the components, or dysfunction of nearby muscles.[6]
Risk factors of late dislocation (after five years) mainly include:[6]
- Female gender
- Younger age at primary hip arthroplasty
- Previous subluxation without complete dislocation
- Previous trauma
- Substantial weight loss
- Recent onset or progression of dementia or a neurological disorder
- Malposition of the cup
- Wear of the liner, particularly when it causes movement of the head of more than 2 mm within the cup compared to its original position
- Prosthesis loosening with migration
Surgeons who perform more of the operations each year tend to have fewer patients dislocate. Doing the surgery from an anterior approach seems to lower dislocation rates when small diameter heads are used, but the benefit has not been shown when compared to modern posterior incisions with the use of larger diameter heads. The use of larger diameter head size does in itself decrease the risk of dislocation, even though this correlation is only found in head sizes up to 28 mm, thereafter no additional decrease in dislocation rate is found.[8] Dual mobility hip replacements reduce the risk of dislocation.[9] People can decrease the risk further by keeping the leg out of certain positions during the first few months after surgery.
Limb length inequality
Most adults prior to a hip replacement have a limb length inequality of 0–2 cm which they were born with and which causes no deficits.[10] It is common for people to feel a limb length inequality after total hip replacement.[11] Sometimes the leg seems long immediately after surgery when in fact both are equal length. An arthritic hip can develop contractures that make the leg behave as if it is short. When these are relieved with replacement surgery and normal motion and function are restored, the body feels that the limb is now longer than it was. This feeling usually subsides by 6 months after surgery as the body adjusts to the new hip joint. The cause of this feeling is variable, and usually related to abductor muscle weakness, pelvic obliquity, and minor lengthening of the hip during surgery (<1 cm) to achieve stability and restore the joint to pre-arthritic mechanics. If the limb length difference remains bothersome to the patient more than 6 months after surgery, a shoe lift can be used. Only in extreme cases is surgery required for correction.
Fracture
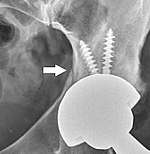
Intraoperative fractures may occur. After surgery, bones with internal fixation devices in situ are at risk of periprosthetic fractures at the end of the implant, an area of relative mechanical stress. Post-operative femoral fractures are graded by the Vancouver classification.
Vein thrombosis
Venous thrombosis such as deep vein thrombosis and pulmonary embolism are relatively common following hip replacement surgery. Standard treatment with anticoagulants is for 7–10 days; however treatment for more than 21 days may be superior.[12][13] Extended-duration anticoagulants (up to 35 days following surgery) may prevent VTE in people undergoing hip replacement surgery.[13] Research from 2013 has on the other hand suggested that anticoagulants in otherwise healthy patients undergoing a so-called fast track protocol with hospital stays under five days, might only be necessary while in the hospital.[14]
Some physicians and patients may consider having an ultrasonography for deep vein thrombosis after hip replacement.[15] However, this kind of screening should only be done when indicated because to perform it routinely would be unnecessary health care.[15]
Intermittent pneumatic compression (IPC) devices are sometimes used for prevention of blood clots following total hip replacement. While there are a variety of different devices, it is currently unclear whether one is more effective than another.[16]
Osteolysis
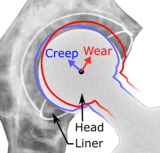
Many long-term problems with hip replacements are the result of osteolysis. This is the loss of bone caused by the body's reaction to polyethylene wear debris, fine bits of plastic that come off the cup liner over time. An inflammatory process causes bone resorption that may lead to subsequent loosening of the hip implants and even fractures in the bone around the implants. In an attempt to eliminate the generation of wear particles, ceramic bearing surfaces are being used in the hope that they will have less wear and less osteolysis with better long-term results. Metal cup liners joined with metal heads (metal-on-metal hip arthroplasty) were also developed for similar reasons. In the lab these show excellent wear characteristics and benefit from a different mode of lubrication. At the same time that these two bearing surfaces were being developed, highly cross linked polyethylene plastic liners were also developed. The greater cross linking significantly reduces the amount of plastic wear debris given off over time. The newer ceramic and metal prostheses do not always have the long-term track record of established metal on poly bearings. Ceramic pieces can break leading to catastrophic failure. This occurs in about 2% of the implants placed. They may also cause an audible, high pitched squeaking noise with activity. Metal-on-metal arthroplasty releases metal debris into the body raising concerns about the potential dangers of these accumulating over time. Highly cross linked polyethylene is not as strong as regular polyethylene. These plastic liners can crack or break free of the metal shell that holds them.
Loosening
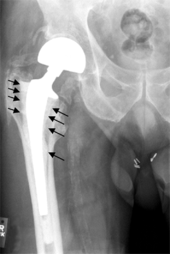
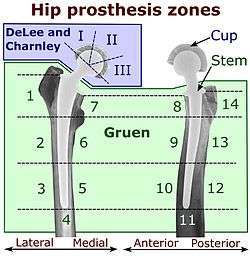
On radiography, it is normal to see thin radiolucent areas of less than 2 mm around hip prosthesis components, or between a cement mantle and bone. However, these may still indicate loosening of the prosthesis if they are new or changing, and areas greater than 2 mm may be harmless if they are stable.[19] The most important prognostic factors of cemented cups are absence of radiolucent lines in DeLee and Charnley zone I, as well as adequate cement mantle thickness.[20] In the first year after insertion of uncemented femoral stems, it is normal to have mild subsidence (less than 10 mm).[19] The direct anterior approach has been shown to itself be a risk factor for early femoral component loosening.[21][22][23]
Metal sensitivity
Concerns are being raised about the metal sensitivity and potential dangers of metal particulate debris. New publications[24][25] have demonstrated development of pseudotumors, soft tissue masses containing necrotic tissue, around the hip joint. It appears these masses are more common in women and these patients show a higher level of iron in the blood. The cause is unknown and is probably multifactorial. There may be a toxic reaction to an excess of particulate metal wear debris or a hypersensitivity reaction to a normal amount of metal debris.
Metal hypersensitivity is a well-established phenomenon and is common, affecting about 10–15% of the population.[26] Contact with metals can cause immune reactions such as skin hives, eczema, redness and itching. Although little is known about the short- and long-term pharmacodynamics and bioavailability of circulating metal degradation products in vivo, there have been many reports of immunologic type responses temporally associated with implantation of metal components. Individual case reports link hypersensitivity immune reactions with adverse performance of metallic clinical cardiovascular, orthopedic and plastic surgical and dental implants.[26]
Metal toxicity
Most hip replacements consist of cobalt and chromium alloys, or titanium. Stainless steel is no longer used. All implants release their constituent ions into the blood. Typically these are excreted in the urine, but in certain individuals the ions can accumulate in the body. In implants which involve metal-on-metal contact, microscopic fragments of cobalt and chromium can be absorbed into the person's bloodstream. There are reports of cobalt toxicity with hip replacement.[27][28]
Nerve palsy
Post operative sciatic nerve palsy is another possible complication. The incidence of this complication is low. Femoral nerve palsy is another but much more rare complication. Both of these will typically resolve over time, but the healing process is slow. Patients with pre-existing nerve injury are at greater risk of experiencing this complication and are also slower to recover.
Chronic pain
A few patients who have had a hip replacement suffer chronic pain after the surgery. Groin pain can develop if the muscle that raises the hip (iliopsoas) rubs against the edge of the acetabular cup. Bursitis can develop at the trochanter where a surgical scar crosses the bone, or if the femoral component used pushes the leg out to the side too far. Also some patients can experience pain in cold or damp weather. Incision made in the front of the hip (anterior approach) can cut a nerve running down the thigh leading to numbness in the thigh and occasionally chronic pain at the point where the nerve was cut (a neuroma).
Death
The rate of perioperative mortality for elective hip replacements is significantly less than 1%.[29][30]
Metal-on-metal hip implant failure
By 2010, reports in the orthopaedic literature increasingly cited the problem of early failure of metal on metal prostheses in a small percentage of patients.[31] Failures may relate to release of minute metallic particles or metal ions from wear of the implants, causing pain and disability severe enough to require revision surgery in 1–3% of patients.[32] Design deficits of some prothesis models, especially with heat-treated alloys and a lack of special surgical experience accounting for most of the failures. In 2010, surgeons at medical centers such as the Mayo Clinic reported reducing their use of metal-on-metal implants by 80 percent over the previous year in favor of those made from other materials, like combinations of metal and plastic.[33] The cause of these failures remain controversial, and may include both design factors, technique factors, and factors related to patient immune responses (allergy type reactions). In the United Kingdom the Medicines and Healthcare Products Regulatory Agency commenced an annual monitoring regime for metal-on-metal hip replacement patients from May 2010.[34] Data which are shown in The Australian Orthopaedic Association's 2008 National Joint replacement registry, a record of nearly every hip implanted in that country over the previous 10 years, tracked 6,773 BHR (Birmingham Hip Resurfacing) Hips and found that less than 0.33% may have been revised due to the patient's reaction to the metal component.[35] Other similar metal-on-metal designs have not fared as well, where some reports show 76% to 100% of the people with these metal-on-metal implants and have aseptic implant failures requiring revision also have evidence of histological inflammation accompanied by extensive lymphocyte infiltrates, characteristic of delayed type hypersensitivity responses.[36] It is not clear to what extent this phenomenon negatively affects orthopedic patients. However, for patients presenting with signs of an allergic reactions, evaluation for sensitivity should be conducted. Removal of the device that is not needed should be considered, since removal may alleviate the symptoms. Patients who have allergic reactions to cheap jewelry are more likely to have reactions to orthopedic implants. There is increasing awareness of the phenomenon of metal sensitivity and many surgeons now take this into account when planning which implant is optimal for each patient.
On March 12, 2012, The Lancet published a study, based on data from the National Joint Registry of England and Wales, finding that metal-on-metal hip implants failed at much greater rates than other types of hip implants and calling for a ban on all metal-on-metal hips.[37] The analysis of 402,051 hip replacements showed that 6.2% of metal-on-metal hip implants had failed within five years, compared to 1.7% of metal-on-plastic and 2.3% of ceramic-on-ceramic hip implants. Each 1 mm (0.039 in) increase in head size of metal-on-metal hip implants was associated with a 2% increase of failure.[38] Surgeons of the British Hip Society are recommending that large head metal-on-metal implants should no longer be performed.[39][40]
On February 10, 2011, the U.S. FDA issued an advisory on metal-metal hip implants, stating it was continuing to gather and review all available information about metal-on-metal hip systems.[41] On June 27–28, 2012, an advisory panel met to decide whether to impose new standards, taking into account findings of the study in The Lancet.[28][42][43] No new standards, such as routine checking of blood metal ion levels, were set, but guidance was updated.[44] Currently, FDA has not required hip implants to be tested in clinical trials before they can be sold in the U.S.[45] Instead, companies making new hip implants only need to prove that they are "substantially equivalent" to other hip implants already on the market. The exception is metal-on-metal implants, which were not tested in clinical trials but because of the high revision rate of metal-on-metal hips, in the future the FDA has stated that clinical trials will be required for approval and that post-market studies will be required to keep metal on metal hip implants on the market.[46]
Modern process

The modern artificial joint owes much to the 1962 work of Sir John Charnley at Wrightington Hospital. His work in the field of tribology resulted in a design that almost completely replaced the other designs by the 1970s. Charnley's design consisted of three parts:
- stainless steel one-piece femoral stem and head
- polyethylene (originally Teflon), acetabular component, both of which were fixed to the bone using
- PMMA (acrylic) bone cement
The replacement joint, which was known as the Low Friction Arthroplasty, was lubricated with synovial fluid. The small femoral head (7⁄8 in (22.2 mm)) was chosen for Charnley's belief that it would have lower friction against the acetabular component and thus wear out the acetabulum more slowly. Unfortunately, the smaller head dislocated more easily. Alternative designs with larger heads such as the Mueller prosthesis were proposed. Stability was improved, but acetabular wear and subsequent failure rates were increased with these designs. The Teflon acetabular components of Charnley's early designs failed within a year or two of implantation. This prompted a search for a more suitable material. A German salesman showed a polyethylene gear sample to Charnley's machinist, sparking the idea to use this material for the acetabular component. The UHMWPE acetabular component was introduced in 1962. Charnley's other major contribution was to use polymethylmethacrylate (PMMA) bone cement to attach the two components to the bone. For over two decades, the Charnley Low Friction Arthroplasty, and derivative designs were the most used systems in the world. It formed the basis for all modern hip implants.
The Exeter hip stem was developed in the United Kingdom during the same time as the Charnley device. Its development occurred following a collaboration between Orthopaedic Surgeon Robin Ling and University of Exeter engineer Clive Lee and it was first implanted at the Princess Elizabeth Orthopaedic Hospital in Exeter in 1970.[48] The Exeter Hip is a cemented device, but with a slightly different stem geometry. Both designs have shown excellent long-term durability when properly placed and are still widely used in slightly modified versions.
Early implant designs had the potential to loosen from their attachment to the bones, typically becoming painful ten to twelve years after placement. In addition, erosion of the bone around the implant was seen on x-rays. Initially, surgeons believed this was caused by an abnormal reaction to the cement holding the implant in place. That belief prompted a search for an alternative method to attach the implants. The Austin Moore device had a small hole in the stem into which bone graft was placed before implanting the stem. It was hoped bone would then grow through the window over time and hold the stem in position. Success was unpredictable and the fixation not very robust. In the early 1980s, surgeons in the United States applied a coating of small beads to the Austin Moore device and implanted it without cement. The beads were constructed so that gaps between beads matched the size of the pores in native bone. Over time, bone cells from the patient would grow into these spaces and fix the stem in position. The stem was modified slightly to fit more tightly into the femoral canal, resulting in the Anatomic Medullary Locking (AML) stem design. With time, other forms of stem surface treatment and stem geometry have been developed and improved.
Initial hip designs were made of a one-piece femoral component and a one-piece acetabular component. Current designs have a femoral stem and separate head piece. Using an independent head allows the surgeon to adjust leg length (some heads seat more or less onto the stem) and to select from various materials from which the head is formed. A modern acetabulum component is also made up of two parts: a metal shell with a coating for bone attachment and a separate liner. First the shell is placed. Its position can be adjusted, unlike the original cemented cup design which are fixed in place once the cement sets. When proper positioning of the metal shell is obtained, the surgeon may select a liner made from various materials.
To combat loosening caused by polyethylene wear debris, hip manufacturers developed improved and novel materials for the acetabular liners. Ceramic heads mated with regular polyethylene liners or a ceramic liner were the first significant alternative. Metal liners to mate with a metal head were also developed. At the same time these designs were being developed, the problems that caused polyethylene wear were determined and manufacturing of this material improved. Highly crosslinked UHMWPE was introduced in the late 1990s. The most recent data comparing the various bearing surfaces has shown no clinically significant differences in their performance. Potential early problems with each material are discussed below. Performance data after 20 or 30 years may be needed to demonstrate significant differences in the devices. All newer materials allow use of larger diameter femoral heads. Use of larger heads significantly decreases the chance of the hip dislocating, which remains the greatest complication of the surgery.
When currently available implants are used, cemented stems tend to have a better longevity than uncemented stems. No significant difference is observed in the clinical performance of the various methods of surface treatment of uncemented devices. Uncemented stems are selected for patients with good quality bone that can resist the forces needed to drive the stem in tightly. Cemented devices are typically selected for patients with poor quality bone who are at risk of fracture during stem insertion. Cemented stems are less expensive due to lower manufacturing cost, but require good surgical technique to place them correctly. Uncemented stems can cause pain with activity in up to 20% of patients during the first year after placement as the bone adapts to the device. This is rarely seen with cemented stems.
Techniques
There are several incisions, defined by their relation to the gluteus medius. The approaches are posterior (Moore), lateral (Hardinge or Liverpool),[49] antero-lateral (Watson-Jones),[50] anterior (Smith-Petersen)[51] and greater trochanter osteotomy. There is no compelling evidence in the literature for any particular approach, but consensus of professional opinion favours either modified anterolateral (Watson-Jones) or posterior approach.
Posterior approach
The posterior (Moore or Southern) approach accesses the joint and capsule through the back, taking piriformis muscle and the short external rotators of the femur. This approach gives excellent access to the acetabulum and femur and preserves the hip abductors and thus minimizes the risk of abductor dysfunction post operatively. It has the advantage of becoming a more extensile approach if needed. Critics cite a higher dislocation rate, although repair of the capsule, piriformis and the short external rotators along with use of modern large diameter head balls reduces this risk. Limited evidence suggests that the posterior approach may cause less nerve damage.[52]
Lateral approach
The lateral approach is also commonly used for hip replacement. The approach requires elevation of the hip abductors (gluteus medius and gluteus minimus) to access the joint. The abductors may be lifted up by osteotomy of the greater trochanter and reapplying it afterwards using wires (as per Charnley), or may be divided at their tendinous portion, or through the functional tendon (as per Hardinge) and repaired using sutures. Although this approach has a lower dislocation risk than the posterior approach, critics note that occasionally the abductor muscles do not heal back on, leading to pain and weakness which is often very difficult to treat.
Antero-lateral approach
The anterolateral approach develops the interval between the tensor fasciae latae and the gluteus medius. The Gluteus medius, gluteus minimus and hip capsule are detached from the anterior (front) for the greater trochanter and femoral neck and then repaired with heavy suture after the replacement of the joint.
Anterior approach
The anterior approach uses an interval between the sartorius muscle and tensor fasciae latae. Dr. Joel Matta and Dr. Bert Thomas have adapted this approach, which was commonly used for pelvic fracture repair surgery, for use when performing hip replacement. When used with older hip implant systems that had a small diameter head, dislocation rates were reduced compared to surgery performed through a posterior approach. With modern implant designs, dislocation rates are similar between the anterior and posterior approaches.[53] The anterior approach has been shown in studies to variably improve early functional recovery, with possible complications of femoral component loosening and early revision compared to other approaches[23][21][54][55][56][57]
Minimally invasive approaches
The dual incision approach and other minimally invasive surgery seeks to reduce soft tissue damage through reducing the size of the incision. However, component positioning accuracy and visualization of the bone structures can be significantly impaired as the approaches get smaller. This can result in unintended fractures and soft tissue injury. The majority of current orthopedic surgeons use a "minimally invasive" approach compared to traditional approaches which were quite large comparatively.
Computer-assisted surgery and robotic surgery techniques are also available to guide the surgeon to provide enhanced accuracy. Several commercial CAS and robotic systems are available for use worldwide. Improved patient outcomes and reduced complications have not been demonstrated when these systems are used when compared to standard techniques.[58][59]
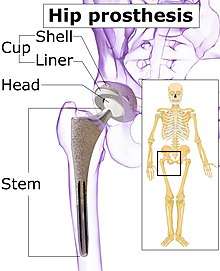
Implants
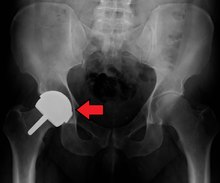
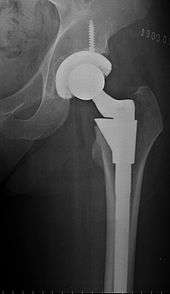
The prosthetic implant used in hip replacement consists of three parts: the acetabular cup, the femoral component, and the articular interface. Options exist for different people and indications. The evidence for a number of newer devices is not very good, including: ceramic-on-ceramic bearings, modular femoral necks, and uncemented monoblock cups.[60] Correct selection of the prosthesis is important.
Acetabular cup
The acetabular cup is the component which is placed into the acetabulum (hip socket). Cartilage and bone are removed from the acetabulum and the acetabular cup is attached using friction or cement. Some acetabular cups are one piece, while others are modular. One-piece (monobloc) shells are either UHMWPE or metal, they have their articular surface machined on the inside surface of the cup and do not rely on a locking mechanism to hold a liner in place. A monobloc polyethylene cup is cemented in place while a metal cup is held in place by a metal coating on the outside of the cup. Modular cups consist of two pieces, a shell and liner. The shell is made of metal; the outside has a porous coating while the inside contains a locking mechanism designed to accept a liner. Two types of porous coating used to form a friction fit are sintered beads and a foam metal design to mimic the trabeculae of cancellous bone and initial stability is influenced by under-reaming and insertion force.[61] Permanent fixation is achieved as bone grows onto or into the porous coating. Screws can be used to lag the shell to the bone providing even more fixation. Polyethylene liners are placed into the shell and connected by a rim locking mechanism; ceramic and metal liners are attached with a Morse taper.
Femoral component
The femoral component is the component that fits in the femur (thigh bone). Bone is removed and the femur is shaped to accept the femoral stem with attached prosthetic femoral head (ball). There are two types of fixation: cemented and uncemented. Cemented stems use acrylic bone cement to form a mantle between the stem and to the bone. Uncemented stems use friction, shape and surface coatings to stimulate bone to remodel and bond to the implant. Stems are made of multiple materials (titanium, cobalt chromium, stainless steel, and polymer composites) and they can be monolithic or modular. Modular components consist of different head dimensions and/or modular neck orientations; these attach via a taper similar to a Morse taper. These options allow for variability in leg length, offset and version. Femoral heads are made of metal or ceramic material. Metal heads, made of cobalt chromium for hardness, are machined to size and then polished to reduce wear of the socket liner. Ceramic heads are more smooth than polished metal heads, have a lower coefficient of friction than a cobalt chrome head, and in theory will wear down the socket liner more slowly. As of early 2011, follow-up studies in patients have not demonstrated significant reductions in wear rates between the various types of femoral heads on the market. Ceramic implants are more brittle and may break after being implanted.
Articular interface
The articular interface is not part of either implant, rather it is the area between the acetabular cup and femoral component. The articular interface of the hip is a simple ball and socket joint. Size, material properties and machining tolerances at the articular interface can be selected based on patient demand to optimise implant function and longevity whilst mitigating associated risks. The interface size is measured by the outside diameter of the head or the inside diameter of the socket. Common sizes of femoral heads are 28 mm (1.1 in), 32 mm (1.3 in) and 36 mm (1.4 in). While 22.25 mm (7⁄8 in) was common in the first modern prostheses, now even larger sizes are available from 38 to over 54 mm. Larger-diameter heads lead to increased stability and range of motion whilst lowering the risk of dislocation. At the same time they are also subject to higher stresses such as friction and inertia. Different combinations of materials have different physical properties which can be coupled to reduce the amount of wear debris generated by friction. Typical pairings of materials include metal on polyethylene (MOP), metal on crosslinked polyethylene (MOXP), ceramic on ceramic (COC), ceramic on crosslinked polyethylene (COXP) and metal on metal (MOM). Each combination has different advantages and disadvantages.
Configuration
Post-operative projectional radiography is routinely performed to ensure proper configuration of hip prostheses.
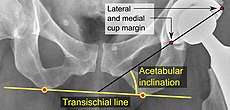
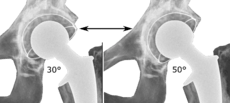
The direction of the acetabular cup influences the range of motion of the leg, and also affects the risk of dislocation.[7] For this purpose, the acetabular inclination and the acetabular anteversion are measurements of cup angulation in the coronal plane and the sagittal plane, respectively.
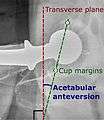 Acetabular anteversion.[63] This parameter is calculated on a lateral radiograph as the angle between the transverse plane and a line going through the (anterior and posterior) margins of the acetabular cup.[63]
Acetabular anteversion.[63] This parameter is calculated on a lateral radiograph as the angle between the transverse plane and a line going through the (anterior and posterior) margins of the acetabular cup.[63]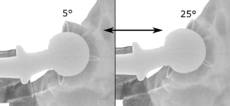
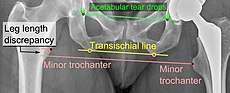
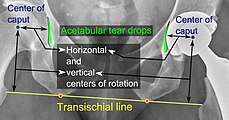 Center of rotation: The horizontal center of rotation is calculated as the distance between the acetabular teardrop and the center of the head (or caput) of the prosthesis and/or the native femoral head on the contralateral side.[62] The vertical center of rotation instead uses the transischial line for reference.[62] The parameter should be equal on both sides.[62]
Center of rotation: The horizontal center of rotation is calculated as the distance between the acetabular teardrop and the center of the head (or caput) of the prosthesis and/or the native femoral head on the contralateral side.[62] The vertical center of rotation instead uses the transischial line for reference.[62] The parameter should be equal on both sides.[62]
Alternatives and variations
Conservative management
The first line approach as an alternative to hip replacement is conservative management which involves a multimodal approach of oral medication, injections, activity modification and physical therapy.[64] Conservative management can prevent or delay the need for hip replacement.
Preoperative care
Preoperative education is currently an important part of patient care. There is some evidence that it may slightly reduce anxiety before hip or knee replacement, with low risk of negative effects.[65]
Hemiarthroplasty
Hemiarthroplasty is a surgical procedure which replaces one half of the joint with an artificial surface and leaves the other part in its natural (pre-operative) state. This class of procedure is most commonly performed on the hip after an intracapsular fracture of the neck of the femur (a hip fracture). The procedure is performed by removing the head of the femur and replacing it with a metal or composite prosthesis. The most commonly used prosthesis designs are the Austin Moore prosthesis and the Thompson Prosthesis. More recently a composite of metal and HDPE which forms two interphases (bipolar prosthesis) has also been used. The monopolar prosthesis has not been shown to have any advantage over bipolar designs. The procedure is recommended only for elderly and frail patients, due to their lower life expectancy and activity level. This is because with the passage of time the prosthesis tends to loosen or to erode the acetabulum.[68] Independently mobile older adults with hip fractures may benefit from a total hip replacement instead of hemiarthroplasty.[69]
 Hip prosthesis for hemiarthroplasty. This example is bipolar, meaning that the head has two separate articulations.
Hip prosthesis for hemiarthroplasty. This example is bipolar, meaning that the head has two separate articulations.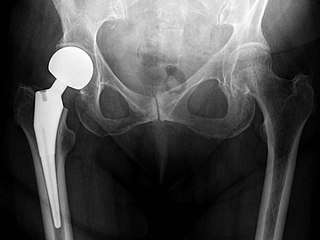 X-ray of the hips, with a right-sided hemiarthroplasty.
X-ray of the hips, with a right-sided hemiarthroplasty.
Hip resurfacing
Hip resurfacing is an alternative to hip replacement surgery. It has been used in Europe for over seventeen years and become a common procedure. Health-related quality of life measures are markedly improved and patient satisfaction is favorable after hip resurfacing arthroplasty.[70]
The minimally invasive hip resurfacing procedure is a further refinement to hip resurfacing.
Viscosupplementation
Current alternatives also include viscosupplementation, or the injection of artificial lubricants into the joint.[71] Use of these medications in the hip is off label. The cost of treatment is typically not covered by health insurance organizations.
Some believe that the future of osteoarthritis treatment is bioengineering, targeting the growth and/or repair of the damaged, arthritic joint. Centeno et al. have reported on the partial regeneration of an arthritic human hip joint using mesenchymal stem cells in one patient.[72] It is yet to be shown that this result will apply to a larger group of patients and result in significant benefits. The FDA has stated that this procedure is being practiced without conforming to regulations, but Centeno claims that it is exempt from FDA regulation. It has not been shown in controlled clinical trials to be effective, and costs over $7,000.
Prevalence and cost
Total hip replacement incidence varies in developed countries between 30 (Romania) and 290 (Germany) procedures per 100,000 population per year.[73] Approximately 0.8% of Americans have undergone the procedure.[74]
According to the International Federation of Healthcare Plans, the average cost of a total hip replacement in 2012 was $40,364 in the United States, $11,889 in the United Kingdom, $10,987 in France, $9,574 in Switzerland, and $7,731 in Spain.[2] In the United States, the average cost of a total hip replacement varies widely by geographic region, ranging from $11,327 (Birmingham, Alabama) to $73,927 (Boston, Massachusetts).[75]
History
The earliest recorded attempts at hip replacement were carried out in Germany in 1891 by Themistocles Gluck (1853–1942),[76][77] who used ivory to replace the femoral head (the ball on the femur), attaching it with nickel-plated screws, plaster of Paris, and glue.[78]
On September 28, 1940 at Columbia Hospital in Columbia, South Carolina, American surgeon Dr. Austin T. Moore (1899–1963)[79] performed the first metallic hip replacement surgery. The original prosthesis he designed was a proximal femoral replacement, with a large fixed head made of the cobalt-chrome alloy Vitallium. It was about a foot in length and bolted to the resected end of the femoral shaft (hemiarthroplasty). A later version, the so-called Austin Moore Prosthesis which was introduced in 1952, is still in use today, although rarely. Like modern hip implants, it is inserted into the medullary canal of the femur, and depends on bone growth through a hole in the stem for long-term attachment.
Other animals
See also
References
- Evans, Jonathan T; Evans, Jonathan P; Walker, Robert W; Blom, Ashley W; Whitehouse, Michael R; Sayers, Adrian (February 2019). "How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up". The Lancet. 393 (10172): 647–654. doi:10.1016/S0140-6736(18)31665-9. PMC 6376618. PMID 30782340.
- "2012 comparative price report" (PDF). International Federation of Health Plans. Retrieved 4 October 2015.
- Smith, TO; Aboelmagd, T; Hing, CB; MacGregor, A (September 2016). "Does bariatric surgery prior to total hip or knee arthroplasty reduce post-operative complications and improve clinical outcomes for obese patients? Systematic review and meta-analysis" (PDF). The Bone & Joint Journal. 98-B (9): 1160–1166. doi:10.1302/0301-620x.98b9.38024. PMID 27587514.
- Bozic, Kevin J; Kurtz, Steven M; Lau, Edmund; Ong, Kevin; Vail, Thomas P; Berry, Daniel J (January 2009). "The Epidemiology of Revision Total Hip Arthroplasty in the United States". The Journal of Bone and Joint Surgery. American Volume. 91 (1): 128–133. doi:10.2106/JBJS.H.00155. PMID 19122087.
- Parvizi, Javad; Zmistowski, Benjamin; Berbari, Elie F.; Bauer, Thomas W.; Springer, Bryan D.; Della Valle, Craig J.; Garvin, Kevin L.; Mont, Michael A.; Wongworawat, Montri D.; Zalavras, Charalampos G. (22 September 2011). "New Definition for Periprosthetic Joint Infection: From the Workgroup of the Musculoskeletal Infection Society". Clinical Orthopaedics and Related Research. 469 (11): 2992–2994. doi:10.1007/s11999-011-2102-9. PMC 3183178. PMID 21938532.
- Daniel J. Berry, Jay Lieberman (2012). Surgery of the Hip. Elsevier Health Sciences. p. 1035. ISBN 9781455727056.
- Iain Watt, Susanne Boldrik, Evert van Langelaan and Robin Smithuis. "Hip - Arthroplasty -Normal and abnormal imaging findings". Radiology Assistant. Retrieved 2017-05-21.CS1 maint: multiple names: authors list (link)
- Hailer, Nils P.; Weiss, Rüdiger J.; Stark, André; Kärrholm, Johan (October 2012). "The risk of revision due to dislocation after total hip arthroplasty depends on surgical approach, femoral head size, sex, and primary diagnosis. An analysis of 78,098 operations in the Swedish Hip Arthroplasty Register". Acta Orthopaedica. 83 (5): 442–448. doi:10.3109/17453674.2012.733919. ISSN 1745-3682. PMC 3488169. PMID 23039167.
- Blakeney, William G.; Epinette, Jean-Alain; Vendittoli, Pascal-André (2019-09-01). "Dual mobility total hip arthroplasty: should everyone get one?". EFORT Open Reviews. 4 (9): 541–547. doi:10.1302/2058-5241.4.180045. ISSN 2396-7544. PMC 6771074. PMID 31598332.
- Knutson, Gary A (2005). "Anatomic and functional leg-length inequality: A review and recommendation for clinical decision-making. Part I, anatomic leg-length inequality: prevalence, magnitude, effects and clinical significance". Chiropractic & Osteopathy. 13 (1): 11. doi:10.1186/1746-1340-13-11. PMC 1232860. PMID 16026625.
- Maloney, William J; Keeney, James A (June 2004). "Leg length discrepancy after total hip arthroplasty". The Journal of Arthroplasty. 19 (4): 108–110. doi:10.1016/j.arth.2004.02.018. PMID 15190563.
- Sobieraj, DM; Lee, S; Coleman, CI; Tongbram, V; Chen, W; Colby, J; Kluger, J; Makanji, S; Ashaye, AO; White, CM (May 15, 2012). "Prolonged versus standard-duration venous thromboprophylaxis in major orthopedic surgery: a systematic review". Annals of Internal Medicine. 156 (10): 720–7. doi:10.7326/0003-4819-156-10-201205150-00423. PMID 22412039.
- Forster, Rachel; Stewart, Marlene (2016-03-30). Cochrane Vascular Group (ed.). "Anticoagulants (extended duration) for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair". Cochrane Database of Systematic Reviews. 3: CD004179. doi:10.1002/14651858.CD004179.pub2. PMID 27027384.
- Jørgensen, Christoffer C.; Jacobsen, Michael K.; Soeballe, Kjeld; Hansen, Torben B.; Husted, Henrik; Kjærsgaard-Andersen, Per; Hansen, Lars T.; Laursen, Mogens B.; Kehlet, Henrik (2013). "Thromboprophylaxis only during hospitalisation in fast-track hip and knee arthroplasty, a prospective cohort study". BMJ Open. 3 (12): e003965. doi:10.1136/bmjopen-2013-003965. ISSN 2044-6055. PMC 3863129. PMID 24334158.
- American Academy of Orthopaedic Surgeons (February 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American Academy of Orthopaedic Surgeons, retrieved 19 May 2013, which cites
- Members of 2007 and 2011 AAOS Guideline Development Work Groups on PE/VTED Prophylaxis; Mont, M; Jacobs, J; Lieberman, J; Parvizi, J; Lachiewicz, P; Johanson, N; Watters, W (Apr 18, 2012). "Preventing venous thromboembolic disease in patients undergoing elective total hip and knee arthroplasty". The Journal of Bone and Joint Surgery. American Volume. 94 (8): 673–4. doi:10.2106/JBJS.9408edit. PMC 3326687. PMID 22517384.
- Zhao, Jin Min; He, Mao Lin; Xiao, Zeng Ming; Li, Ting Song; Wu, Hao; Jiang, Hua (2014-12-22). Cochrane Vascular Group (ed.). "Different types of intermittent pneumatic compression devices for preventing venous thromboembolism in patients after total hip replacement". Cochrane Database of Systematic Reviews (12): CD009543. doi:10.1002/14651858.CD009543.pub3. PMID 25528992.
- John J. Callaghan, Aaron G. Rosenberg, Harry E. Rubash (2007). The Adult Hip, Volume 1. Lippincott Williams & Wilkins. p. 958. ISBN 978-0-7817-5092-9.CS1 maint: multiple names: authors list (link)
- Neumann, Daniel R.P.; Thaler, Christoph; Hitzl, Wolfgang; Huber, Monika; Hofstädter, Thomas; Dorn, Ulrich (2010). "Long-Term Results of a Contemporary Metal-on-Metal Total Hip Arthroplasty". The Journal of Arthroplasty. 25 (5): 700–708. doi:10.1016/j.arth.2009.05.018. ISSN 0883-5403. PMID 19596544.
- Roth, Trenton D.; Maertz, Nathan A.; Parr, J. Andrew; Buckwalter, Kenneth A.; Choplin, Robert H. (2012). "CT of the Hip Prosthesis: Appearance of Components, Fixation, and Complications". RadioGraphics. 32 (4): 1089–1107. doi:10.1148/rg.324115183. ISSN 0271-5333. PMID 22786996.
- Steffen Breusch, Henrik Malchau (2005). The Well-Cemented Total Hip Arthroplasty: Theory and Practice. Springer Science & Business Media. p. 336. ISBN 978-3-540-24197-3.
- Eto, Shuichi; Hwang, Katherine; Huddleston, James I.; Amanatullah, Derek F.; Maloney, William J.; Goodman, Stuart B. (March 2017). "The Direct Anterior Approach is Associated With Early Revision Total Hip Arthroplasty". The Journal of Arthroplasty. 32 (3): 1001–1005. doi:10.1016/j.arth.2016.09.012. PMID 27843039.
- Angerame, Marc R.; Fehring, Thomas K.; Masonis, John L.; Mason, J. Bohannon; Odum, Susan M.; Springer, Bryan D. (February 2018). "Early Failure of Primary Total Hip Arthroplasty: Is Surgical Approach a Risk Factor?". The Journal of Arthroplasty. 33 (6): 1780–1785. doi:10.1016/j.arth.2018.01.014. PMID 29439894.
- Meneghini, R. Michael; Elston, Addison S.; Chen, Antonia F.; Kheir, Michael M.; Fehring, Thomas K.; Springer, Bryan D. (January 2017). "Direct Anterior Approach". The Journal of Bone and Joint Surgery. 99 (2): 99–105. doi:10.2106/JBJS.16.00060. PMID 28099299.
- Pandit H, Glyn-Jones S, McLardy-Smith P, et al. (July 2008). "Pseudotumours associated with metal-on-metal hip resurfacings". J Bone Joint Surg Br. 90 (7): 847–51. doi:10.1302/0301-620X.90B7.20213. PMID 18591590.
- Boardman DR, Middleton FR, Kavanagh TG (March 2006). "A benign psoas mass following metal-on-metal resurfacing of the hip". J Bone Joint Surg Br. 88 (3): 402–4. doi:10.1302/0301-620X.88B3.16748. PMID 16498023.
Korovessis P, Petsinis G, Repanti M, Repantis T (June 2006). "Metallosis after contemporary metal-on-metal total hip arthroplasty. Five to nine-year follow-up". J Bone Joint Surg Am. 88 (6): 1183–91. doi:10.2106/JBJS.D.02916. PMID 16757749. - Hallab N, Merritt K, Jacobs JJ (March 2001). "Metal sensitivity in patients with orthopaedic implants". J Bone Joint Surg Am. 83-A (3): 428–36. doi:10.2106/00004623-200103000-00017. PMID 11263649.
- http://www.epi.hss.state.ak.us/bulletins/docs/b2010_14.pdf
- "FDA seeks more advice on metal hip implants". Reuters. 29 March 2012. Retrieved 20 May 2012.
- Coté, John (July 22, 2007). "Hip replacement is not viewed as high-risk surgery; Death is rare, but underlying medical condition a factor". San Francisco Chronicle.
- Medscape Conference Coverage, American Academy of Orthopaedic Surgeons (AAOS) 2009 Annual Meeting, AAOS 2009: Certain Factors Increase Risk for Death After Total Hip Arthroplasty, Barbara Boughton, March 3, 2009.
- Mikael, Mark M.; Hanssen, Arlen D.; Sierra, Rafael J. (2009). "Failure of Metal-on-Metal Total Hip Arthroplasty Mimicking Hip Infection". The Journal of Bone and Joint Surgery. American Volume. 2009 (91): 443–446. doi:10.2106/JBJS.H.00603. PMID 19181991.
- Meier, Barry (March 3, 2010). "As Use of Metal-on-Metal Hip Implants Grows, Studies Raise Concerns". The New York Times.
- Meier, Barry (March 3, 2010). "Concerns Over 'Metal on Metal' Hip Implants". The New York Times.
- "Medical Device Alert: All metal-on-metal (MoM) hip replacements". Medicines and Healthcare products Regulatory Agency. 22 April 2010. MDA/2010/033. Archived from the original on 25 April 2010. Retrieved 2010-05-07. Cite journal requires
|journal=(help) - Table HT 46. Australian Orthopaedic Association National Joint Replacement Registry Annual Report. Adelaide: AOA; 2008
- Milosev I, Trebse R, Kovac S, Cör A, Pisot V (June 2006). "Survivorship and retrieval analysis of Sikomet metal-on-metal total hip replacements at a mean of seven years". J Bone Joint Surg Am. 88 (6): 1173–82. doi:10.2106/JBJS.E.00604. PMID 16757748.
- Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW (March 2012). "Failure rates of stemmed metal-on-metal hip replacements: analysis of data from the National Joint Registry of England and Wales". Lancet. 379 (9822): 1199–204. doi:10.1016/S0140-6736(12)60353-5. PMID 22417410.
- Gallagher, James (13 March 2012). "Metal-on-metal hip replacements 'high failure rate'". BBC. Retrieved 20 May 2012.
- Pijls, B. G.; Meessen, J. M. T. A.; Schoones, J. W.; Fiocco, M.; Heide, H. J. L. van der; Sedrakyan, A.; Nelissen, R. G. H. H. (2016). "Increased Mortality in Metal-on-Metal versus Non-Metal-on-Metal Primary Total Hip Arthroplasty at 10 Years and Longer Follow-Up: A Systematic Review and Meta-Analysis". PLOS ONE. 11 (6): e0156051. Bibcode:2016PLoSO..1156051P. doi:10.1371/journal.pone.0156051. ISSN 1932-6203. PMC 4905643. PMID 27295038.
- Roberts, Michelle (5 March 2012). "Surgeons call for end to metal hip replacements". BBC. Retrieved 20 May 2012.
- "Metal-on-Metal Hip Implants". Food and Drug Administration. February 10, 2011. Retrieved January 4, 2012.
- "Orthopaedic and Rehabilitation Devices Panel of the Medical Devices Advisory Committee Meeting Announcement". Food and Drug Administration. 27 March 2012. FDA-2012-N-0293. Retrieved 20 May 2012.
- FDA Executive Summary Memorandum – Metal-on-Metal Hip Implant System (PDF) (Report). Food and Drug Administration. 27 June 2012. Retrieved 15 March 2013.
- "Concerns about Metal-on-Metal Hip Implants". Food and Drug Administration. 17 January 2013. Retrieved 15 March 2013.
- "Study Suggests Women Have Higher Risk of Hip Implant Failure - For The Media - JAMA Network". media.jamanetwork.com.
- Rising, Joshua P.; Reynolds, Ian S.; Sedrakyan, Art (2012). "Delays and Difficulties in Assessing Metal-on-Metal Hip Implants". New England Journal of Medicine. 367 (1): e1. doi:10.1056/NEJMp1206794. PMID 22716934.
- Andrew Still (2002-11-02). "Total Hip Replacement". University of Southern California. Retrieved 2017-01-05.
- Timperley, A John (20 October 2017). "Robin Ling obituary". The Guardian. Retrieved 22 October 2017.
- Pai VS (1997). "A comparison of three lateral approaches in primary total hip replacement". Int Orthop. 21 (6): 393–398. doi:10.1007/s002640050193. PMC 3619565. PMID 9498150. Archived from the original on 2002-01-08.
- "Anterolateral Approach to Hip Joint: (Watson Jones) – Wheeless' Textbook of Orthopaedics". Retrieved 2007-11-26.
- "Anterior Approach to the Hip (Smith Petersen) – Wheeless' Textbook of Orthopaedics". Retrieved 2007-11-26.
- Jolles, Brigitte M.; Bogoch, Earl R. (2006-07-19). "Posterior versus lateral surgical approach for total hip arthroplasty in adults with osteoarthritis". Cochrane Database of Systematic Reviews (3): CD003828. doi:10.1002/14651858.cd003828.pub3. ISSN 1465-1858. PMID 16856020.
- Maratt, Joseph D.; Gagnier, Joel J.; Butler, Paul D.; Hallstrom, Brian R.; Urquhart, Andrew G.; Roberts, Karl C. (September 2016). "No Difference in Dislocation Seen in Anterior Vs Posterior Approach Total Hip Arthroplasty". The Journal of Arthroplasty. 31 (9): 127–130. doi:10.1016/j.arth.2016.02.071. PMID 27067754.
- Christensen, Christian P.; Jacobs, Cale A. (September 2015). "Comparison of Patient Function during the First Six Weeks after Direct Anterior or Posterior Total Hip Arthroplasty (THA): A Randomized Study". The Journal of Arthroplasty. 30 (9): 94–97. doi:10.1016/j.arth.2014.12.038. PMID 26096071.
- Higgins, Brendan T.; Barlow, Daniel R.; Heagerty, Nathan E.; Lin, Tim J. (March 2015). "Anterior vs. Posterior Approach for Total Hip Arthroplasty, a Systematic Review and Meta-analysis". The Journal of Arthroplasty. 30 (3): 419–434. doi:10.1016/j.arth.2014.10.020. PMID 25453632.
- Meermans, G.; Konan, S.; Das, R.; Volpin, A.; Haddad, F. S. (June 2017). "The direct anterior approach in total hip arthroplasty". The Bone & Joint Journal. 99-B (6): 732–740. doi:10.1302/0301-620X.99B6.38053. PMID 28566391.
- Graves, Sara C.; Dropkin, Benjamin M.; Keeney, Benjamin J.; Lurie, Jon D.; Tomek, Ivan M. (30 November 2015). "Does Surgical Approach Affect Patient-reported Function After Primary THA?". Clinical Orthopaedics and Related Research. 474 (4): 971–981. doi:10.1007/s11999-015-4639-5. PMC 4773324. PMID 26620966.
- Parsley, Brian S. (February 2018). "Robotics in Orthopedics: A Brave New World". The Journal of Arthroplasty. 33 (8): 2355–2357. doi:10.1016/j.arth.2018.02.032. PMID 29605151.
- Jacofsky, David J.; Allen, Mark (October 2016). "Robotics in Arthroplasty: A Comprehensive Review". The Journal of Arthroplasty. 31 (10): 2353–2363. doi:10.1016/j.arth.2016.05.026. PMID 27325369.
- Nieuwenhuijse, M. J.; Nelissen, R. G. H. H.; Schoones, J. W.; Sedrakyan, A. (9 September 2014). "Appraisal of evidence base for introduction of new implants in hip and knee replacement: a systematic review of five widely used device technologies". BMJ. 349 (sep09 1): g5133. doi:10.1136/bmj.g5133. PMC 4159610. PMID 25208953.
- Amirouche, Farid (2014). "Factors influencing initial cup stability in total hip arthroplasty". Clinical Biomechanics. 29 (10): 1177–85. doi:10.1016/j.clinbiomech.2014.09.006. PMID 25266242.
- Vanrusselt, Jan; Vansevenant, Milan; Vanderschueren, Geert; Vanhoenacker, Filip (2015). "Postoperative radiograph of the hip arthroplasty: what the radiologist should know". Insights into Imaging. 6 (6): 591–600. doi:10.1007/s13244-015-0438-5. ISSN 1869-4101. PMC 4656234. PMID 26487647.
- Shin, W. C.; Lee, S. M.; Lee, K. W.; Cho, H. J.; Lee, J. S.; Suh, K. T. (2015). "The reliability and accuracy of measuring anteversion of the acetabular component on plain anteroposterior and lateral radiographs after total hip arthroplasty". The Bone & Joint Journal. 97-B (5): 611–616. doi:10.1302/0301-620X.97B5.34735. ISSN 2049-4394. PMID 25922453.
- Cibulka MT, White DM, Woehrle J, et al. (April 2009). "Hip pain and mobility deficits—hip osteoarthritis: clinical practice guidelines linked to the international classification of functioning, disability, and health from the orthopaedic section of the American Physical Therapy Association". J Orthop Sports Phys Ther. 39 (4): A1–25. doi:10.2519/jospt.2009.0301. PMC 3963282. PMID 19352008.
- McDonald, S; Page, MJ; Beringer, K; Wasiak, J; Sprowson, A (2014). "Preoperative education for hip or knee replacement". The Cochrane Database of Systematic Reviews (published 13 May 2014) (5): CD003526. doi:10.1002/14651858.CD003526.pub3. PMID 24820247.
- Jones, Carl; Briffa, Nikolai; Jacob, Joshua; Hargrove, Richard (2017). "The Dislocated Hip Hemiarthroplasty: Current Concepts of Etiological factors and Management". The Open Orthopaedics Journal. 11 (Suppl-7, M4): 1200–1212. doi:10.2174/1874325001711011200. ISSN 1874-3250. PMC 5721319. PMID 29290857.
- Ninh, Christopher C.; Sethi, Anil; Hatahet, Mohammed; Les, Clifford; Morandi, Massimo; Vaidya, Rahul (2009). "Hip Dislocation After Modular Unipolar Hemiarthroplasty". The Journal of Arthroplasty. 24 (5): 768–774. doi:10.1016/j.arth.2008.02.019. ISSN 0883-5403. PMID 18555648.
- van der Meulen, M.C.H.; Allen, W.A.; Giddings, V.L.; Athanasiou, K.A.; Poser, R.D.; Goodman, S.B.; Smith, R.L.; Beaupré, G.S. "Effect of hemiarthroplasty on acetabular cartilage". 1996 Project Reports. VA Palo Alto Health Care System's Bone and Joint Rehabilitation Research and Development Center.
- Metcalfe, David (2019). "Total hip arthroplasty versus hemiarthroplasty for independently mobile older adults with intracapsular hip fractures". BMC Musculoskeletal Disorders. 20 (11): 226. doi:10.1186/s12891-019-2590-4. PMC 6525472. PMID 31101041.
- Koutras C, Antoniou SA, Talias MA, Heep H (19 May 2015). "Impact of Total Hip Resurfacing Arthroplasty on Health-Related Quality of Life Measures: A Systematic Review and Meta-Analysis". J Arthroplasty. 30 (11): 1938–52. doi:10.1016/j.arth.2015.05.014. PMID 26067708.
- van den Bekerom MP, Lamme B, Sermon A, Mulier M (August 2008). "What is the evidence for viscosupplementation in the treatment of patients with hip osteoarthritis? Systematic review of the literature". Arch Orthop Trauma Surg. 128 (8): 815–823. doi:10.1007/s00402-007-0447-z. PMID 17874246.
- Centeno CJ, Kisiday J, Freeman M, Schultz JR (July 2006). "Partial regeneration of the human hip via autologous bone marrow nucleated cell transfer: A case study". Pain Physician. 9 (3): 253–6. PMID 16886034. Archived from the original on 2009-02-12.
- Kurtz SM, Ong KL, Lau E, Widmer M, Maravic M, Gómez-Barrena E, de Pina Mde F, Manno V, Torre M, Walter WL, de Steiger R, Geesink RG, Peltola M, Röder C (2011). "International survey of primary and revision total knee replacement". Int Orthop. 35 (12): 1783–9. doi:10.1007/s00264-011-1235-5. PMC 3224613. PMID 21404023.
- Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, Jiranek WA, Berry DJ (2015). "Prevalence of Total Hip and Knee Replacement in the United States". J Bone Joint Surg Am. 97 (17): 1386–97. doi:10.2106/JBJS.N.01141. PMC 4551172. PMID 26333733.
- "A study of cost variations for knee and hip replacement surgeries in the U.S." (PDF). Blue Cross Blue Shield Association. 21 January 2015. Archived from the original (PDF) on 22 October 2015. Retrieved 4 October 2015.
- "History of Artificial Joints - ppt video online download". slideplayer.com.
- Brand, RA; Mont, MA; Manring, M (2011). "Biographical sketch: Themistocles Gluck (1853–1942)". Clin. Orthop. Relat. Res. 469 (6): 1525–1527. doi:10.1007/s11999-011-1836-8. PMC 3094624. PMID 21403990.
- Gomez PF; Morcuende JA (2005). "Early attempts at hip arthroplasty—1700s to 1950s". Iowa Orthop J. 25: 25–9. PMC 1888777. PMID 16089067.
- "What You Need to Know About Joint Replacement Surgery". about.com.
.jpg)