Cyhalothrin
Cyhalothrin is an organic compound that is used as a pesticide.[1] It is a pyrethroid, a class of synthetic insecticides that mimic the structure and insecticidal properties of the naturally occurring insecticide pyrethrin which comes from the flowers of chrysanthemums. Pyrethroids such as cyhalothrin are often preferred as an active ingredient in insecticides because they remain effective for longer periods of time than pyrethrin. It is a colorless solid, although samples can appear beige, with a mild odor. It has a low water solubility and is nonvolatile. It is used to control insects in cotton crops.[2]
 | |
| Names | |
|---|---|
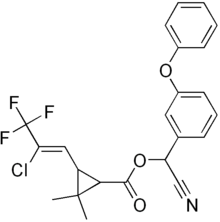
| IUPAC name
3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethyl-cyano(3-phenoxyphenyl)methyl cyclopropanecarboxylate | |
| Other names
Cyhalothrine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.062.209 |
| KEGG | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C23H19ClF3NO3 |
| Molar mass | 449.85 g·mol−1 |
| Appearance | Dark brown/green solid; colorless when pure |
| Density | 1.33 ± 0.03 g/cm3 |
| Melting point | 49.2 °C (120.6 °F; 322.3 K) |
| Boiling point | 498.9 °C (930.0 °F; 772.0 K) decomposes (760 mmHg) |
Solubility in water |
0.005 mg/l [20 °C] |
| Solubility in other solvents | Very soluble in xylene; soluble in ethyl acetate, diethyl ether, cyclohexane, methanol |
| Acidity (pKa) | 5.43 (decomposes in alkaline solutions) |
| Pharmacology | |
| QP53AC06 (WHO) | |
| Hazards | |
EU classification (DSD) (outdated) |
|
| NFPA 704 (fire diamond) | |
| Flash point | 185 °C (365 °F; 458 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
Lambda-cyhalothrin is a mixture of isomers of cyhalothrin.[3] Syngenta held the patent for lambda-cyhalothrin which expired in most major markets in 2003.[4]
Gamma-cyhalothrin is a single stereoisomer of the pyrethroid insecticide cyhalothrin, and as such shares physical, chemical, and biological properties with both cyhalothrin and lambda-cyhalothrin, which are mixtures of 4 and 2 isomers respectively. Gamma-cyhalothrin is the most insecticidally active isomer of cyhalothrin/lambda-cyhalothrin, and thus the technical gamma-cyhalothrin product may be considered a refined form of cyhalothrin/lambda-cyhalothrin in that it has been purified by removal of less active and inactive isomers. Thus, similar levels of insecticidal efficacy for gamma-cyhalothrin can be obtained with significantly reduced application rates as compared with either cyhalothrin or lambda-cyhalothrin. The tolerance under 40 CFR 180.438 currently identifies lambda-cyhalothrin as a 1:1 mixture of two isomers and their epimers, one of which is the gamma isomer. The gamma isomer is present at 42% in this mixture. In contrast, a proposed tolerance would entail having the gamma isomer present at 98%.[5]
Mechanism of action
Pyrethroids, including lambda-cyhalothrin, disrupt the functioning of the nervous system in an organism. By disrupting the nervous system of insects, lambda-cyhalothrin may cause paralysis or death. Temperature influences its effectiveness. It is highly toxic to many fish and aquatic invertebrate species. Bioconcentration is possible in aquatic species, but bioaccumulation is not likely. Binding of lambda-cyhalothrin to soil and sediment reduces exposure and may lessen the risk to fish. Field studies found no significant adverse effects to fish. Lambda-cyhalothrin is also highly toxic to bees, although again field studies found few effects. In laboratory studies, alkaline water degraded lambda-cyhalothrin with an approximate half-life of 7 days, although at neutral and acidic pHs, degradation did not occur. Sunlight accelerates degradation in water and soil. The half-life of lambda-cyhalothrin on plant surfaces is 5 days. Lambda-cyhalothrin has a low potential to contaminate ground water due to its low water solubility and high potential to bind to soil.[6]
Safety
The LD50 is 79–56 mg/kg (rats, oral).[2] It decomposes in extreme heat (257 °C),[7] liberating toxic gases like CO and HCN.[8][9] It is a potent irritant to mucous membranes and is highly toxic in concentrations higher than 97%.[10]
Brands
In the United States, 'Triazicide' and 'Hot Shot' are used in the home landscape and garden markets.[11][12][13] Other brand names include 'Danger', 'Karate', 'Kung-fu', 'Matador', 'Cyzmic CS', and 'Demand CS'; Proaxis in Indonesia by PT Dow AgroSciences Indonesia. Spectrum Brands sells lambda-cyhalothrin insecticides under the brand names Real Kill and Spectracide Bug Off, and also carries a gamma-cyhalothrin version named Spectracide Triazicide. Terro also makes an outdoor soil-insect-prevention treatment similar to Home Defense using this ingredient. Lambda-cyhalothrin is also marketed as Charge, Cutter Backyard Bug Control, Demand CS, Excaliber, Grenade, Hallmark, Icon CS, Karate, Matador, OMS 0321, PP321, Saber, Samurai, and Sentinel.
References
- "Cyhalothrin". Cameo Chemicals. United States National Oceanic and Atmospheric Administration. Retrieved 2008-05-08.
- Robert L. Metcalf "Insect Control" in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi:10.1002/14356007.a14_263
- "Lambda-cyhalothrin factsheet" (PDF). National Pesticide Telecommunications Network (Oregon State University and the U.S. Environmental Protection Agency). Retrieved 2008-04-15.
- "WHO Specifications and Evaluations for Public Health Pesticides" (PDF). WHO. Retrieved 2011-06-10.
- "Lambda-Cyhalothrin and an Isomer Gamma-Cyhalothrin; Tolerances for Residues" (PDF). US EPA FEDERAL REGISTER, April 8, 2004 (Volume 69, Number 68). Retrieved 2012-09-07.
- "Lambda-cyhalothrin(General Fact Sheet)" (PDF). NPIC. Retrieved 2012-09-07.
- MSDS of Lambda Cyhalothrin
- "Safety Data Sheet" (PDF). Nufarm. 2012-06-19.
- "MATERIAL SAFETY DATA SHEET" (PDF).
- "Lambda-cyhalothrin" (PDF). NPIC.
- "Insecticides in the Home Landscape and Garden". Iowa State University Department of Entomology. Retrieved 2009-04-29.
- "Regulatory Decision Document: Lambda-Cyhalothrin Demand CS Insecticide". Health Canada. Archived from the original on 2012-07-30. Retrieved 2011-06-10.
- "Demand CS". Syngenta. Retrieved 2011-06-10.
External links
- Gamma-cyhalothrin in the Pesticide Properties DataBase (PPDB)
- Lambda-cyhalothrin in the Pesticide Properties DataBase (PPDB)
- Righi, D. Abbud; Palermo-Neto, J. (2003-09-01). "Behavioral effects of type II pyrethroid cyhalothrin in rats". Toxicology and Applied Pharmacology. 191 (2): 167–76. doi:10.1016/S0041-008X(03)00236-9. PMID 12946652. Retrieved 2008-05-08.
- "Cyhalothrin/Karate (CASRN 68085-85-8)". IRIS. United States Environmental Protection Agency. 10 Jan 2008. Retrieved 2008-05-08.
