VFC Publications: Supplement
Last updated April 23, 2014
Appendix: Methods for the cost-benefit analyses presented in "Benefits from Immunization during the Vaccines for Children Program Era — United States, 1994–2013", MMWR 2014;63:352-5.
Decision Analysis Model
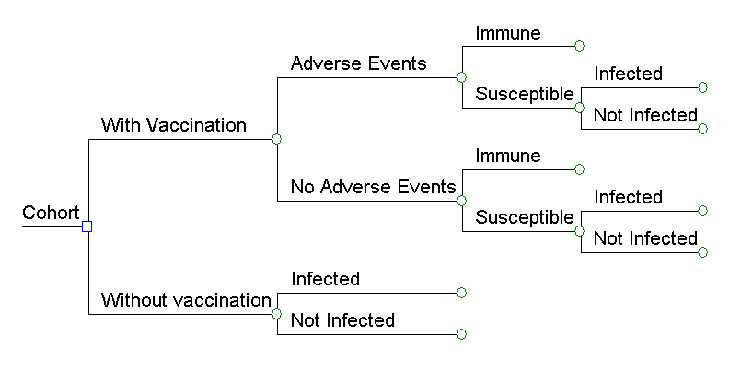
We developed one decision tree for each vaccine as the basis for our model (see example, Figure 1) and then evaluated the effect of routine childhood vaccination on hypothetical US birth cohorts for children born during 1994 through 2013 (range 3,744,999 children to 4,147,947 children per cohort) [http://www.census.gov/popest/] from birth through death. When the VFC program began in 1994, vaccines targeting nine diseases were provided for children aged ≤6 years: diphtheria, tetanus, and pertussis (DTaP), polio (OPV then IPV), Haemophilus influenzae type b disease (Hib), hepatitis B (Hep B), and measles, mumps, and rubella (MMR). Three vaccines were added between 1996 and 2013: varicella (1996), pneumococcal disease (PCV; 7-valent in 2000, 13-valent in 2010), and rotavirus vaccine (Rota; 2006); influenza and hepatitis A vaccines were also added by VFC during this period but were not included in our model. In the 2013 schedule, the Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP) recommended routine administration of 5 doses of DTaP, 3 or 4 doses of Hib (depending on product used), 4 doses of IPV, 2 doses of MMR, 3 doses of HepB, 2 doses of VAR, 4 doses of PCV13, and 2 or 3 doses of Rota (depending on product used) by age 6.[1] Our analysis is based on coverage attained for each of these vaccines in the United States each year during 1994-2012 as estimated by the National Immunization Survey (NIS),[2] Immunization Information Systems[3,4] available in some areas, and School and Childcare Vaccination Surveys.[5]
The analyses were performed from 2 perspectives: direct cost (direct medical and nonmedical costs) and societal (direct and indirect costs). Direct medical costs include those associated with treating an initial infection as well as costs associated with complications and sequelae of diseases. Direct non-medical costs include travel costs, costs for special education of children disabled by diseases, and costs for other supplies for special needs. Indirect costs include the productivity losses due to premature mortality and permanent disability among cohort members as well as opportunity costs associated with parents who miss work to care for their sick children or cohort members themselves who miss work due to vaccine-preventable illness. Benefits of routine childhood immunization are quantified as the savings in direct and indirect costs that accrue from averting morbidity and mortality by vaccination. The costs associated with the immunization program include the vaccines, their administration, parent travel and work time lost and adverse events associated with these vaccines. All costs were adjusted to 2013 dollars using general and medical Consumer Price Indices, and all costs and benefits in the future were discounted at a 3% annual rate. We calculated net present values (NPV) for all vaccines together. NPV is the sum of the discounted benefits from the routine childhood immunization program minus the sum of the discounted costs.
The data for burden of diseases, costs of diseases, costs for outbreak control and costs of vaccination and adverse events used in our analysis were compiled from a variety of sources: the published literature including surveillance data, study data, and expert consensus; several large computerized data sets; and CDC unpublished data. When it was necessary to make estimates about the incidence of disease and complications from multiple publications, results from existing meta-analyses were used.
Top of PageEstimating the burden of diseases without vaccination
The age-specific annual incidence rates of diphtheria, tetanus, pertussis, Hib, poliomyelitis, measles, mumps, rubella and varicella diseases, and the prevalence, complications and perinatal transmission of hepatitis B in the United States in the pre-vaccine era were obtained from a previous analysis (Table 1).[6-23]
For pneumococcus--related diseases, the age-specific estimated incidence rates in the United States in the pre-vaccine era were obtained from CDC's Active Bacterial Core Surveillance (ABCs) (for invasive pneumococcal diseases, or IPD) and the literature (for pneumonia and acute otitis media).[24-27] Age-specific pre-vaccine IPD rates and case-fatality rates for IPD were based on data from the ABCs program for 1998 and 1999. All-cause pneumonia incidence and acute otitis media rates were obtained from the literature. [24-27]
For rotavirus disease, we assumed that the cumulative incidence of rotavirus gastroenteritis is 75% in the first 5 years of life,[28-31] the cumulative incidence of hospitalization visits due to rotavirus gastroenteritis in the first 5 years is 1.70%, the cumulative incidence of outpatient visits is 11.14%, the cumulative incidence of emergency department visits is 5.36%, and the cumulative incidence of deaths is 0.00078%.[31]
Top of PageEstimating the burden of diseases with vaccination
For all diseases except varicella, hepatitis B, pneumococcal diseases, and rotavirus, we used surveillance data for 1994-2012 from the National Notifiable Diseases Surveillance System (NNDSS) to estimate the burden of diseases with vaccination in 1994-2013. For varicella, we used age-specific incidence data from West Philadelphia Varicella Active Surveillance Project (VASP) site for 1996-2010, and for 2011 and 2012, we used the age-specific data from 31 states from NNDSS, to estimate the total number of varicella cases in the United States. Based on data from VASP, we assumed that 0-92.3% of reported cases involved persons that had previously received varicella vaccine, with the proportion vaccinated varying by year, and that cases in vaccinated persons were much milder than cases among unvaccinated persons.[32] For hepatitis B, since chronic cases were not reported to NNDSS, we used an established hepatitis B decision analysis model[33] the vaccination coverage rates[2] and the vaccine efficacy estimates[34] to estimate the likelihood of hepatitis B infection and sequelae among vaccinated and unvaccinated children in the cohort. For pneumococcal disease, vaccination era IPD rates and case-fatality rates for IPD were based on data for 2001-2012 from ABCs. All-cause pneumonia incidence and acute otitis media rates were obtained from the literature.[24-27,35] For rotavirus disease, we developed a decision analysis model using the vaccination coverage rates from NIS[2] and efficacies of the two vaccines[36-40] to estimate the likelihood of rotavirus infections and their sequelae among vaccinated children in the cohort.
Top of PageCosts associated with disease
Direct Costs
Direct costs for outbreak control, and outpatient and inpatient visits were included in the analysis. The cost of outpatient visits, average duration of hospital stay, hospitalization costs and costs for outbreak control for each condition related to these diseases including congenital rubella syndrome (CRS), were obtained from published and unpublished studies (Table 2).[7-10,25-27,30,31,33,41-46] All costs shown here were in 2013 dollars.
Indirect Costs
To estimate the productivity losses from premature mortality, we used the human capital approach.[47] Costs for work loss were determined by the number of days of missed work (for provision of care to sick children, for illness among cohort members, or for resulting disability) multiplied by the daily wage rate associated with the value of lost wage-earning work and the imputed value of housekeeping and home-care activities. We assumed the days of morbidity were distributed randomly throughout the week.
Vaccination coverage, costs and adverse events associated with vaccination
Vaccination coverage was based on 1994-2012 NIS data (2012 data were used for 2013), and used to calculate the vaccine costs for each cohort. Overall, more than 50% of US childhood vaccines were publicly purchased in 2013 (CDC, unpublished data, 2013). The 1994-2013 public and private prices for all vaccines were obtained from the CDC Vaccine Price List (https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html). We assumed that the overall rate of vaccine wastage (public and private sectors) was 5%.[48] The federal excise tax that supports the National Vaccine Injury Compensation Program was not included in vaccine prices.
NIS data indicate that more than 70% of children obtained their vaccines from private providers.[49] The cost for administering a vaccine dose during a visit to a private clinic was estimated at $29.07.[9,10] For the public clinic, we used an administration cost of $8.15.[9,10]
We assumed that caregivers take 2 hours off from work to take the child for vaccination (as per previous economic studies[9,10]). We assumed that the average cost for these caregivers was $18.19 per hour, and cost for caregiver’s travel to the clinic was $23.45.[31]
The severe and mild adverse reaction rates of DTaP, Hib, MMR and VAR from the previous analyses were used.[6,23] We assumed that there were no serious side effects for IPV.[50] For HepB, we assumed 1.1 episode of anaphylaxis per 1,000,000 vaccinated children.[51,52] For PCV7, we assumed that 5 per 1,000,000 vaccinated children will have a seizure.[53] For RV, we assumed that 2,250 per 1,000,000[54] vaccinated infants will have physician visits for adverse events and 10 per 1,000,000 vaccinated infants will have intussusception caused by rotavirus vaccine, and that the case-fatality rate for intussusception is 0.4%.
Top of PageTable 1. Annual incidence rates (cases per 100,000) of diphtheria, tetanus, pertussis, Hib, polio, measles, mumps, rubella, hepatitis B, varicella, invasive pneumococcal disease (IPD), and rotavirus before and after vaccine introduction.*
| Without vaccination (i.e., prevaccine era) |
With vaccination (i.e., after vaccine introduction) | |||
|---|---|---|---|---|
| Disease | Baseline rates | Data source (s) | Range of disease rates | Data source(s) |
| Diphtheria† | 600 | 7 | 0 | NNDSS |
| Tetanus† | 0.3 | 7 | 0-0.01 | NNDSS |
| Pertussis† | 4,720 | 7 | 3-43 | NNDSS |
| Hib‡ | 158 | 9 | 0.2-0.3 | ABCs |
| Polio, Paralytic† | 31 | 6 | 0 | NNDSS |
| Measles§ | 10,641 | 10 | 0-3 | NNDSS |
| Rubella† | 6,205 | 10 | 2-27 | NNDSS |
| Mumps† | 3,300 | 10 | 0-1 | NNDSS |
| Hepatitis Bll | 72 | 33 | 6-46 | Modeled |
| Varicellall | 9,839 | 22 | 30-1,140 | VASP and NNDSS |
| Invasive Pneumococcal Disease‡ | 212 | 26 | 14-69 | ABCs |
| Rotavirus*** | 12,750 | 31 | 4,551-7,150 | Modeled |
* Incidence estimates used in the analysis varied by age.
† Estimates shown are for children 5-9 years old.
‡ Estimates shown are for children 1 year old.
§ Estimates shown are for children 2-4 years old.
ll Estimates shown are for children 1-4 years old.
** Estimates shown are for children 2 years old in one region.
*** Estimates shown are for children 2 years old.
Table 2. Probabilities and costs of hospitalizations, and outpatient visits for selected vaccine preventable diseases. All costs are in 2013 US $.*7,12-15,-23,29-31,33,39,40
| Disease | Probability of Hospitalization | No. of Hospitalization Days | Cost per Hospitalization | Cost per outpatient visit |
|---|---|---|---|---|
| Diphtheria | 100% | 6.1 | $16,982 | $100 |
| Tetanus | 100% | 16.7 | $102,584 | $100 |
| Pertussis | 0.65-30% | 5.5-15 | $10,765-22,410 | $100-173 |
| Hib | ||||
|
50-100% | 2-7.29 | $4,111-38,270 | $100-353 |
|
5-30% | 2.84-26.75 | $18,195-49,236 | $310-570 |
| Poliomyelitis | 5-100% | 4-17 | $7,781-50,554 | $100 |
| Measles | 11-100% | 1.3-10.9 | $4,032-46,060 | $88-526 |
| Mumps | 1-100% | 2.8-8.7 | $11,196-46,060 | $110-556 |
| Rubella | 0.1-100% | 2.6-8.7 | $4,886-46,060 | $89-651 |
| Congenital rubella syndrome | ||||
|
100% | 13.6 | $62,233 | $110 |
|
100% | 8.9 | $37,082 | |
|
100% | 2.2 | $8,786 | |
| Hepatitis B | 0.001-100% | 3.9-11 | $15,662-27,051 | $214-599 |
| Varicella | 0.1-2.1% | 3.1-9.3 | $4,136-22,113 | $83-254 |
| Pneumococcal Diseases | 0-100% | 6.4-16.8 | $3,798-25,848 | $86-272 |
| Rotavirus | 0.5-3.8% | 2-3.4 | $3,195-4,793 | $135-455 |
* Some estimates used in the analysis varied by age, outcome of disease, and with or without vaccination program.
Top of PageFigure 1. Simplified Decision Tree
References
- Akinsanya-Beysolow I, Jenkins R, Meissner HC. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for persons aged 0 through 18 years--United States, 2013. MMWR Surveill Summ. 2013; 62 Suppl 1:2-8.
- National, state, and local area vaccination coverage among children aged 19-35 months - United States, 2012. MMWR Morb Mortal Wkly Rep. 2013; 62:733-740.
- Rotavirus vaccination coverage among infants aged 5 months - immunization information system sentinel sites, United States, June 2006-June 2009. MMWR Morb Mortal Wkly Rep. 2010; 59:521-524.
- Lopez AS, Cardemil C, Pabst LJ, Cullen KA, Leung J, Bialek SR. Two-dose varicella vaccination coverage among children aged 7 years--six sentinel sites, United States, 2006-2012. MMWR Morb Mortal Wkly Rep. 2014; 63:174-177.
- Vaccination coverage among children in kindergarten - United States, 2012-13 school year. MMWR Morb Mortal Wkly Rep. 2013; 62:607-612.
- Zhou F, Santoli J, Messonnier ML, Yusuf HR, Shefer A, Chu SY et al. Economic evaluation of the 7-vaccine routine childhood immunization schedule in the United States, 2001. Arch Pediatr Adolesc Med. 2005; 159:1136-1144.
- Ekwueme DU, Strebel PM, Hadler SC, Meltzer MI, Allen JW, Livengood JR. Economic evaluation of use of diphtheria, tetanus, and acellular pertussis vaccine or diphtheria, tetanus, and whole-cell pertussis vaccine in the United States, 1997. Arch Pediatr Adolesc Med. 2000; 154:797-803.
- Cochi SL, Broome CV, Hightower AW. Immunization of US children with Hemophilus influenzae type b polysaccharide vaccine. A cost-effectiveness model of strategy assessment. JAMA. 1985; 253:521-529.
- Zhou F, Bisgard KM, Yusuf HR, Deuson RR, Bath SK, Murphy TV. Impact of universal Haemophilus influenzae type b vaccination starting at 2 months of age in the United States: an economic analysis. Pediatrics. 2002; 110:653-661.
- Zhou F, Reef S, Massoudi M, et al. An economic analysis of the current universal 2-dose MMR Vaccination program in the United States. J Infect Dis. 2004; 189:s131-s145.
- Centers for Disease Control and Prevention. Epidemiology and Prevention for Vaccine-Preventable Disease. Atkinson W, Wolfe S, Hamborsky J, McIntyre L. eds. 11th ed. Washington DC: Public Health Foundation, 2009.
- Murata R. Immunization against diphtheria in Japan. Jpn J Med Sci Biol. 1981; 34:329-354.
- Romanus V, Jonsell R, Bergquist SO. Pertussis in Sweden after the cessation of general immunization in 1979. Pediatr Infect Dis J. 1987; 6:364-371.
- Collins SA. Age incidence of the common communicable diseases in children. Public Health Rep. 1929; 44:763-826.
- Granoff DM, Basden M. Haemophilus influenzae infections in Fresno County, California: a prospective study of the effects of age, race, and contact with a case on incidence of disease. J Infect Dis. 1980; 141:40-46.
- Langmuir A.D. Medical importance of measles. Am J Dis Child. 1962; 103:224-226.
- Centers for Disease Control and Prevention. Summary of Notifiable Disease, United States, 1990. MMWR Morb Mortal Wkly Rep. 1991; 39 (53):53-61.
- Chandran A, Watt JP, Santosham M. Haemophilus influenzae vaccines. In: Plotkin S, Orenstein WA, Offit PA, editors. Vaccines. Elsevier Saunders, 2008: 157-176.
- Vitek CR, Wharton M. Diphtheria Toxoid. In: Plotkin S, Orenstein WA, Offit PA, editors. Elsevier Saunders, 2008: 139-156.
- Wassilak SG, Roper MH, Kretsinger K, Orenstein WA. Tetanus Toxoid. In: Plotkin S, Orenstein WA, Offit PA, editors. Elsevier Saunders, 2008: 805-839.
- Edwards KM, Decker MD. Pertussis Vaccine. In: Plotkin S, Orenstein WA, Offit PA, editors. Elsevier Saunders, 2008: 467-517.
- Zhou F, Ortega-Sanchez IR, Guris D, Shefer A, Lieu T, Seward JF. An economic analysis of the universal varicella vaccination program in the United States. J Infect Dis. 2008; 197 Suppl 2:S156-S164.
- Zhou F, Shefer A, Wenger J, Messonnier M, Wang LY, Lopez A et al. Economic evaluation of the routine childhood immunization program in the United States, 2009. Pediatrics. 2014; 133:577-585.
- Pneumonia hospitalizations among young children before and after introduction of pneumococcal conjugate vaccine--United States, 1997-2006. MMWR Morb Mortal Wkly Rep. 2009; 58:1-4.
- Ray GT, Whitney CG, Fireman BH, Ciuryla V, Black SB. Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr Infect Dis J. 2006; 25:494-501.
- Ray GT, Pelton SI, Klugman KP, Strutton DR, Moore MR. Cost-effectiveness of pneumococcal conjugate vaccine: an update after 7 years of use in the United States. Vaccine. 2009; 27:6483-6494.
- Lieu TA, Ray GT, Black SB, Butler JC, Klein JO, Breiman RF et al. Projected cost-effectiveness of pneumococcal conjugate vaccination of healthy infants and young children. JAMA. 2000; 283:1460-1468.
- Gurwith M, Wenman W, Hinde D, Feltham S, Greenberg H. A prospective study of rotavirus infection in infants and young children. J Infect Dis. 1981; 144:218-224.
- Rodriguez WJ, Kim HW, Brandt CD, Schwartz RH, Gardner MK, Jeffries B et al. Longitudinal study of rotavirus infection and gastroenteritis in families served by a pediatric medical practice: clinical and epidemiologic observations. Pediatr Infect Dis J. 1987; 6:170-176.
- Tucker AW, Haddix AC, Bresee JS, Holman RC, Parashar UD, Glass RI. Cost-effectiveness analysis of a rotavirus immunization program for the United States. JAMA. 1998; 279:1371-1376.
- Widdowson MA, Meltzer MI, Zhang X, Bresee JS, Parashar UD, Glass RI. Cost-effectiveness and potential impact of rotavirus vaccination in the United States. Pediatrics. 2007; 119:684-697.
- Chaves SS, Zhang J, Civen R, Watson BM, Carbajal T, Perella D et al. Varicella disease among vaccinated persons: clinical and epidemiological characteristics, 1997-2005. J Infect Dis. 2008; 197 Suppl 2:S127-S131.
- Margolis HS, Coleman PJ, Brown RE, Mast EE, Sheingold SH, Arevalo JA. Prevention of hepatitis B virus transmission by immunization. An economic analysis of current recommendations. JAMA. 1995; 274:1201-1208.
- Andre FE, Zuckerman AJ. Review: protective efficacy of hepatitis B vaccines in neonates. J Med Virol. 1994; 44:144-151.
- Griffin MR, Moore MR, Whitney CG, Grijalva CG. Continued decline in pneumonia hospitalizations in young children following transition from PCV7 to PCV13 in Tennessee. [Abstract ISPPD-0336]. Pneumonia. 2014; 3:247.
- Vesikari T, Matson DO, Dennehy P, van DP, Santosham M, Rodriguez Z et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006; 354:23-33.
- Boom JA, Tate JE, Sahni LC, Rench MA, Hull JJ, Gentsch JR et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010; 125:e199-e207.
- Staat MA, Payne DC, Donauer S, Weinberg GA, Edwards KM, Szilagyi PG et al. Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics. 2011; 128:e267-e275.
- Cortese MM, LeBlanc J, White K, et al. Leveraging state immunization information systems to measure the effectiveness of rotavirus vaccine. Pediatrics. 2012; In Press.
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006; 354:11-22.
- White CC, Koplan JP, Orenstein WA. Benefits, risks and costs of immunization for measles, mumps and rubella. Am J Public Health. 1985; 75:739-744.
- Hatziandreu EJ, Palmer CS, Halpern MT, and Brown RE. A cost benefit analysis of the OPV vaccine: Report prepared for the Centers for Disease Control and Prevention. Report , Battelle Inc, Arlington, VA. 1994.
- Miller MA, Sutter RW, Strebel PM, Hadler SC. Cost-effectiveness of incorporating inactivated poliovirus vaccine into the routine childhood immunization schedule. JAMA. 1996; 276:967-971.
- Boyle CA, Decoufle P, Yeargin-Allsopp M. Prevalence and health impact of developmental disabilities in US children. Pediatrics. 1994; 93:399-403.
- Parker AA, Staggs W, Dayan GH, Ortega-Sanchez IR, Rota PA, Lowe L et al. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med. 2006; 355:447-455.
- Bonebrake AL, Silkaitis C, Monga G, Galat A, Anderson J, Trad JT et al. Effects of mumps outbreak in hospital, Chicago, Illinois, USA, 2006. Emerg Infect Dis. 2010; 16:426-432.
- Haddix, A. E., Teutch, S. M., and Corso, P. S. Prevention Effectiveness: A Guide to Decision Analysis and Economic Evaluation. New York, NY: Oxford University Press. 2003.
- Setia S, Mainzer H, Washington ML, Coil G, Snyder R, Weniger BG. Frequency and causes of vaccine wastage. Vaccine. 2002; 20:1148-1156.
- Groom H, Kolasa M, Wooten K, Ching P, Shefer A. Childhood immunization coverage by provider type. J Public Health Manag Pract. 2007; 13:584-589.
- Update: vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1996; 45:1-35.
- Bohlke K, Davis RL, Marcy SM, Braun MM, DeStefano F, Black SB et al. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics. 2003; 112:815-820.
- DiMiceli L, Pool V, Kelso JM, Shadomy SV, Iskander J. Vaccination of yeast sensitive individuals: review of safety data in the US vaccine adverse event reporting system (VAERS). Vaccine. 2006; 24:703-707.
- Wise RP, Iskander J, Pratt RD, Campbell S, Ball R, Pless RP et al. Postlicensure safety surveillance for 7-valent pneumococcal conjugate vaccine. JAMA. 2004; 292:1702-1710.
- Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009; 58:1-25.
- Page last reviewed: April 23, 2014
- Page last updated: April 23, 2014
- Content source:


 ShareCompartir
ShareCompartir