Diagnosis
Clinicians: For 24/7 diagnostic assistance, specimen collection guidance, shipping instructions, and treatment recommendations, please contact the CDC Emergency Operations Center at 770-488-7100. More detailed guidance is under Information for Public Health & Medical Professionals.
Print-and-Go Fact Sheet
Initial testing: Cerebrospinal fluid (CSF) studies of patients infected with Naegleria fowleri typically demonstrate a pattern similar to bacterial meningitis with an elevated opening pressure, a polymorphonuclear pleocytosis, normal or low glucose, and elevated protein. However the observations of blood in the CSF and/or motile ameba are clues to a potential diagnosis of PAM.
Case definition 1: Laboratory-confirmed Naegleria fowleri infection is defined as the detection of Naegleria fowleri:
- Organisms in CSF, biopsy, or tissue specimens, or
- Nucleic acid in CSF, biopsy, or tissue specimens, or
- Antigen in CSF, biopsy, or tissue specimens
Tests available: Diagnostic testing is not widely available for PAM. Clinicians who suspect PAM should contact their state health department and/or CDC (24/7 Emergency Operation Center—770-488-1700). CDC can assist with diagnosis and provide treatment recommendations. Telediagnosis can be arranged at CDC by emailing photos through DPDx, CDC’s Division of Parasitic Diseases and Malaria telediagnosis tool. Instructions for submitting photos through DPDx are available at the DPDx Contact Us page.
Diagnostic Tests
Direct Visualization
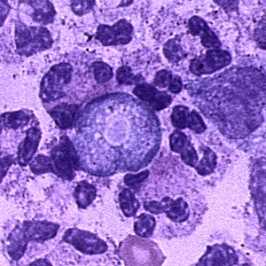
Trophozoite of Naegleria fowleri in CSF, stained with H&E.
The diagnosis of Naegleria fowleri infection can be made most quickly by microscopic examination of fresh, unfrozen, unrefrigerated cerebrospinal fluid (CSF) (NOTE: samples cannot be frozen or refrigerated because cold temperatures kill the amebae). A wet mount of freshly-centrifuged CSF sediment might demonstrate actively moving trophozoites. Naegleria fowleri (15-30 µm trophozoite) moves rapidly (~1 µm/s) using eruptive pseudopods and moves sinuously in a generally linear forward direction. Additionally, Naegleria can be identified in CSF smears or cultures using hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), trichrome, Giemsa, or Wright-Giemsa stains. A Gram stain should be avoided as the amebae can be destroyed during heat fixation. A stained CSF smear will show ameboid trophozoites with morphology typical of Naegleria (i.e., a nucleus with a large, centrally located and densely staining nucleolus). If amebae are identified in the CSF, the diagnosis of PAM should be subsequently confirmed with PCR or immunohistochemical (IHC) tests.
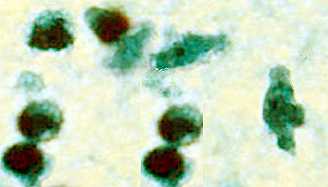
Trophozoite of Naegleria fowleri in CSF, stained with trichrome. Image courtesy of the Texas State Health Department.
The diagnosis can also be made from microscopic examination of hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), trichrome, Giemsa, or Wright-Giemsa stained smears of brain biopsy or autopsy specimens, which might demonstrate trophozoites with morphology typical of Naegleria fowleri. The ameboid trophozoites measure 10-35 µm but when rounded are usually 10-15 µm in diameter. The cytoplasm is granular and contains many vacuoles. The single nucleus is large and has a large, dense karyosome. Naegleria fowleri does not form cysts in human tissues.
References
- Visvesvara GS. Amebic meningoencephalitides and keratitis: challenges in diagnosis and treatment. Curr Opin Infect Dis. 2010 Dec;23(6):590-4.
- da Rocha-Azevedo B, Tanowitz HB, Marciano-Cabral F. Diagnosis of infections caused by pathogenic free-living amoebae. Interdiscip Perspect Infect Dis. 2009;2009:251406.
Immunohistochemical Staining
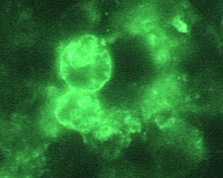
Indirect Immunofluorescence (IIF) assay for Naegleria fowleri, 1000x oil magnification
Immunohistochemical staining techniques, such as indirect immunofluorescent (IIF) staining and immune alkaline phosphatase staining (IHC), use an antibody specific for Naegleria fowleri followed by microscopic examination to identify Naegleria fowleri in tissue, culture, or CSF 1, 2.
References
- Visvesvara GS. Amebic meningoencephalitides and keratitis: challenges in diagnosis and treatment. Curr Opin Infect Dis. 2010 Dec;23(6):590-4.
- da Rocha-Azevedo B, Tanowitz HB, Marciano-Cabral F. Diagnosis of infections caused by pathogenic free-living amoebae. Interdiscip Perspect Infect Dis. 2009;2009:251406.
Serology
Serologic testing for Naegleria fowleri using indirect immunofluorescent antibody (IFA) testing is currently considered a research technique as it has not been evaluated for use as a routine diagnostic procedure. Although IFA can be performed to measure serum antibody titers in patient sera, most patients with PAM die before an immune response is mounted. However, the California PAM survivor did exhibit a serologic response 1,2.
References
- Seidel JS, Harmatz P, Visvesvara GS, Cohen A, Edwards J, Turner J. Successful treatment of primary amebic meningoencephalitis. N Engl J Med. 1982;306:346-8.
- Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50:1-26.
Polymerase chain reaction (PCR)
DNA has been successfully amplified from CSF and unfixed tissue samples. Typing of isolates can be done but little is known about the natural populations to put these data in a biological context. An increasing number of PCR-based techniques (conventional and real-time PCR) have been described for detection and identification of free-living amebic infections in clinical specimens, but are only available in selected reference diagnostic laboratories 1-3. A real-time PCR was developed at CDC for qualitative assessment of Naegleria fowleri, Acanthamoeba spp ., and Balamuthia mandrillaris in clinical samples. This assay uses distinct primers and TaqMan probes for the simultaneous identification of all three free-living amebae 1.
References
- Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol. 2006;44(10):3589-95.
- Robinson BS, Monis PT, Dobson PJ. Rapid, sensitive, and discriminating identification of Naegleria spp. by real-time PCR and melting-curve analysis. Appl Environ Microbiol. 2006;72(9):5857-63.
- Marciano-Cabral F, MacLean R, Mensah A, LaPat-Polasko L. Identification of Naegleria fowleri in domestic water sources by nested PCR. Appl Envirol Microbiol. 2003;69:5864-9.
Culture
Culture is a routine procedure used to identify free-living amebae in clinical and environmental specimens. It involves inoculating mammalian cell cultures and monitoring for cytopathogenicity or growth on E. coli lawns. For growing on an E. coli lawn, the sample is added to a growth plate covered in bacteria that can serve as a food source for Naegleria fowleri. The initial screening is accomplished by incubating the plate at a higher temperature (108°F/42°C) that kills most free-living amebae, while selecting for thermophilic amebae, such as Naegleria fowleri or other amebae. This initial screen shows up as tracks made by an ameba as it moves across the plate eating the bacteria. If there are no amebae on the plate grown at the higher temperature, then Naegleria fowleri is not present. If thermophilic amebae are present on the plate grown at the higher temperature, then these amebae undergo further specific testing to determine whether Naegleria fowleri is present since other thermophilic free-living amebae could be present. Naegleria can be specifically identified in cultures from clinical specimens using hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), trichrome, Giemsa, or Wright-Giemsa stains. Stained cultures might demonstrate trophozoites with morphology typical of Naegleria fowleri, as previously described. In culture, trophozoites may measure more than 40 µm. A negative culture result does not rule out the presence of free-living amebae and other tests should be performed 1.
References
Specimens Needed for Pre-Mortem Diagnosis
Clinical Specimens for Diagnosis at CDC
If possible, CDC requests that the following specimens be sent for diagnostic testing at CDC:
- Fresh CSF (Please DO NOT FREEZE and DO NOT REFRIGERATE as this kills the amebae)
- If the patient has had a biopsy, we also request:
- Fresh brain tissue (Please DO NOT FREEZE and DO NOT REFRIGERATE);
- Formalin-fixed and paraffin embedded tissues
- Three stained H&E slides
More Information
Specimens Needed for Post-Mortem and Autopsy Diagnosis
To better understand the pathogenesis of PAM and the potential for transmission via organ transplantation 2 (See Naegleria fowleri Organ Transplantation), CDC would like to encourage autopsies for PAM case patients whose families consent.
CNS Tissue
Naegleria fowleri is most likely detected in biopsy or autopsy tissue collected from the area surrounding the nasal-olfactory bulbs in the brain. However, CDC requests that tissues be collected from other CNS sites in addition to the olfactory bulb to look for other possible locations of ameba entry into the brain, such as around the auditory nerve.
Extra-CNS Tissue
All possible steps should be taken to minimize the possibility of cross-tissue contamination between CNS and extra-CNS tissues. These steps should, at a minimum, include:
-
- Completing the gross examination and sample collection from all extra-CNS tissues prior to examination of the CNS tissues
- Utilizing separate workspaces and dissecting tools for the extra-CNS and CNS tissues
- Placing recovered samples of extra-CNS and CNS tissues in separate formalin containers
- Processing all tissues, particularly extra-CNS and CNS, separately
- Cutting extra-CNS and CNS tissues separately
- If the same equipment is used to cut the tissue, cut extra-CNS tissues first and include a cleaning step in between different tissues
Specimens can then be sent to CDC.
Clinical Specimens for Diagnosis at CDC
If possible, please send the following specimens:
- Fresh CSF (Please DO NOT FREEZE and DO NOT REFRIGERATE as this kills the amebae)
- Fresh, unfixed brain tissue
- Fresh, unfixed tissue (other than brain)
- Formalin-fixed, paraffin-embedded, tissue
- Three H&E-stained slides
- Six unstained slides
- Paraffin-embedded tissue block
- Photos of gross brain morphology
- Particularly around olfactory and auditory areas
- Serum
References
- Council for State and Territorial Epidemiologists (CSTE). Case Definitions for Non-notifiable Infections Caused by Free-living Amebae (Naegleria fowleri, Balamuthia mandrillaris, and Acanthamoeba spp.) [PDF – 10 pages]. Infectious Disease Committee. 2012.
- Roy SL, Metzger R, Chen JG, Laham FR, Martin M, Kipper SW, Smith LE, Lyon GM 3rd, Haffner J, Ross JE, Rye AK, Johnson W, Bodager D, Friedman M, Walsh DJ, Collins C, Inman B, Davis BJ, Robinson T, Paddock C, Zaki SR, Kuehnert M, DaSilva A, Qvarnstrom Y, Sriram R, Visvesvara GS. Risk for transmission of Naegleria fowleri from solid organ transplantation. Am J Transplant. 2014;14(1):163-71.
- Page last reviewed: July 27, 2017
- Page last updated: July 27, 2017
- Content source:


 ShareCompartir
ShareCompartir
