WHO Western Pacific Region (WPR) 2014-2015
In fiscal year 2015, the U.S. Centers for Disease Control and Prevention (CDC) funded six bilateral influenza cooperative agreements in the Western Pacific Region of Asia. These agreements are with Ministries of Health or institutions designated by the Ministry of Health to work with the Centers for Disease Control and Prevention (CDC) to build capacity to routinely identify, diagnose, and respond to seasonal and pandemic influenza.
Direct Country Support
Direct country support through non-research cooperative agreements is established in the following six countries/entities:
- Cambodia [204 KB, 2 pages]
- China [349 KB, 3 pages]
- Mongolia [155 KB, 3 pages]
- Secretariat of the Pacific Community (SPC) [129 KB, 3 pages]
- Phillipines [158 KB, 2 pages]
- Vietnam [349 KB, 3 pages]
In addition, CDC supports the World Health Organization (WHO) Regional Office for the Western Pacific through a cooperative agreement; this agreement provide assistance to three additional countries:
- Fiji
- Lao People's Democratic Republic [265 KB, 3 pages]
- Papua New Guinea (PNG)
Core Activities
The core activities of the bilateral agreements and technical assistance are:
- To build sustainable national capacity for surveillance for seasonal influenza, pandemic influenza and other emerging diseases and preparedness for implementation of the International Health Regulations 2005 (IHR).
- To make routine contributions of surveillance data to the WHO Global Influenza Surveillance and Response System (GISRS).
- To increase the geographic reach of WHO’s GISRS.
- To provide quicker access to critical virus isolates from humans and birds for WHO GISRS.
- To increase the numbers of shipments and influenza isolates provided by WHO WPR influenza laboratories to WHO Collaborating Centers (CC) for analysis.
- To develop sustainable epidemiologic and virologic surveillance systems for severe influenza in order to gain an understanding of the burden of disease in the WHO WPR.
In fiscal year 2013, CDC expanded its cooperative agreement portfolio to include a Vaccine Policy component. Country support was established in Vietnam and China to introduce or expand the use of seasonal influenza vaccines.
The core activities include: conducting a needs assessment to identify barriers, developing a three-year action plan to introduce vaccines, implementing the plan and introducing or expanding vaccine use to the target population through a national policy.
Influenza Division Contacts
Vashonia Smith, MPA
Public Health Advisor
Extramural Program
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Atlanta, GA
Email: vdw8@cdc.gov
Tomas Rodriguez, MA (until April 2015)
Public Health Advisor
Extramural Program
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Atlanta, GA
Email: trr0@cdc.gov
Danielle Iuliano, MPH, PhD
Senior Research Epidemiologist
International Epidemiology and Research Team
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Atlanta, GA
Email: aoi0@cdc.gov
Andrew Corwin, PhD
QED–Contractor
U.S. Embassy
Vientiane, Lao People’s Democratic Republic
Email: corwinal@state.gov
James Kile, DVM, MPH (until July 2015)
Director, Influenza and Animal-Human Interface
Program
CDC, Vietnam
Hanoi, Vietnam
Email: jkile@cdc.gov
Carolyn Greene, MD
Influenza Program Director
CDC, Beijing
Beijing, China
Email: cqg4@cdc.gov
WHO Regional Office for the Western Pacific (WPRO)
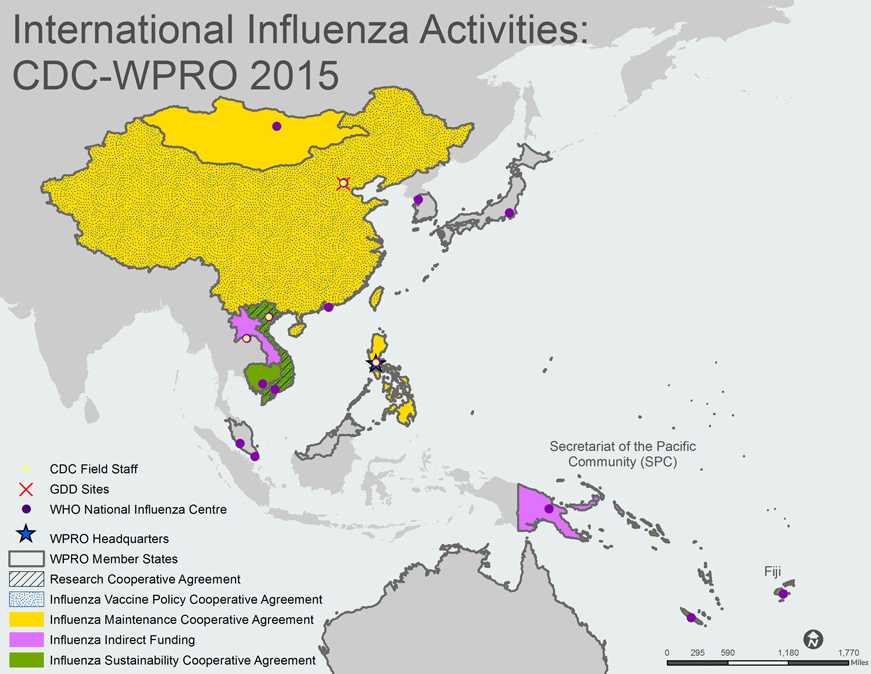
A map of the WHO Western Pacific Region (WPR) shows 12 of the 37 WPR member states/countries. The member countries shown, outlined with gray borders, include Australia, Cambodia, China, Japan, Lao PDR, Malaysia, Mongolia, Papua New Guinea, Philippines, Singapore, South Korea, and Vietnam.
Countries with green shading indicate that the Influenza Division provides project funding and technical assistance through Sustainability Cooperative Agreements. Cambodia, Secretariat of the Pacific Community (SPC), and Vietnam are shaded green on the map. Fiji, Lao PDR and Papua New Guinea are shaded pink to indicate that they receive indirect funding from the Influenza Division. Vietnam has black diagonal stripes to indicate that they have a Research Cooperative Agreement. China and Vietnam are also shaded with blue dots to indicate that they have Vaccine Policy Cooperative Agreements. China, Mongolia, and Philippines are shaded yellow to indicate Maintenance Cooperative Agreements.
CDC Influenza Division Field Staff, indicated by a yellow dot outlined in red, are located in the following cities: Beijing, Hanoi, Manila, and Vientiane.
The Global Disease Detection [GDD] Site, indicated by a red “X”, is located in Beijing.
WHO National Influenza Centers (NICs), indicated by purple dots, are located in the following cities: Beijing, Goroka, Hanoi, Ho Chi Minh City, Hong Kong, Kuala Lumpur, Manila, Phnom Penh, Singapore, Taipei, Tokyo, Ulaanbaatar and Vientiane.
The WHO Regional Office of the Western Pacific (WPRO), indicated by a blue star, is located in Manila, Philippines.
Highlights
- Assisted with the response to the second (2013–2014) and third (2014–2015) waves of human infection with avian influenza A(H7H9) in China, through routine risk assessments and three-level (Country Office, Regional Office, Headquarters) and tripartite (FAO, OIE, WHO) teleconferences.
- Maintained hotlines for event-based surveillance (EBS) in Lao PDR and Cambodia.
- Initiated severe acute respiratory infection (SARI) surveillance in intensive care units (ICU) in Fiji.
- Implemented an Early Warning and Response System to monitor potential communicable disease outbreaks following Cyclone Pam in Vanuatu.
- Integrated animal surveillance into EBS in Lao PDR and coordinated joint human/animal avian influenza coordination meetings.
- Integrated influenza surveillance into the Ministry of Health national plan in Fiji.
- Worked with WHO Collaborating Centers (CC), and other laboratory experts to improve testing proficiency for emerging infectious diseases (EID) in the Region.
U.S. CDC Direct Support
The five-year cooperative agreement between CDC and WHO WPRO began on September 30, 2011. It supported the implementation of the Asia Pacific Strategy for Emerging Diseases (APSED 2010), which provides a common framework to strengthen national and regional capacities to manage emerging diseases and public health threats, improve pandemic influenza preparedness and comply with the core capacity requirements of the International Health Regulations (2005). The strategy includes components (such as surveillance and laboratory strengthening) that support the Global Influenza Surveillance and Response System (GISRS), which, in this region, currently consists of 21 National Influenza Centres (NICs) in 15 countries, three WHO Collaborating Centres (CCs) for Reference and Research on Influenza in Australia, China, and Japan, two Essential Regulatory Laboratories in Australia and Japan and an H5 Reference Laboratory in Hong Kong, China. The cooperative agreement also includes funds directed to countries through WHO country offices in Cambodia, China, Pacific Island Countries, Lao People's Democratic Republic (Lao PDR), and Papua New Guinea (PNG).
Surveillance
Event-and indicator-based surveillance was supported in Lao PDR and Cambodia. These systems supported the successful deployment of Rapid Response Teams for several outbreak investigations. An Early Warning and Response System was established in Vanuatu after Cyclone Pam to monitor potential communicable disease outbreaks. Improved reporting and information sharing was also supported.
Cambodia began publishing monthly respiratory disease bulletins based on influenza-like illness (ILI) and severe acute respiratory infection (SARI) data.
Lao PDR produces a weekly laboratory report based on ILI and SARI sentinel site surveillance in 13 provincial hospitals. The Pacific Island Countries released their first influenza bulletin integrating syndromic and virologic data from ILI and laboratory surveillance sites.
Annual meetings of the National Influenza Centres (NIC) and influenza surveillance sites were held in Beijing, China in November 2013 and in Jakarta, Indonesia in August 2014. These meetings brought together representatives of NICs and influenza surveillance staff from WHO’s Western Pacific and South East Asia regions as well as representatives from the WHO CC’s in the USA, Australia, Japan, China and Hong Kong (China).
The 2013 meeting was attended by 119 participants from 22 countries and focused on lessons learned from avian influenza A(H7N9) as well as experiences for severe respiratory infection detection. The 2014 meeting focused on ways to further strengthen influenza surveillance systems, data reporting, response and the role of GISRS to provide data for vaccine policy development.
Support was also provided to Papua New Guinea to create a position dedicated to collecting respiratory samples from ill persons and improving surveillance efficiency, and to send PNG NIC staff for training at the WHO CC at the Victorian Infectious Diseases Reference Laboratory (VIDRL) in Melbourne. Support was also provided to the WHO Country Office for the ESR Team Lead.
Surveillance Activities
2014
- Facilitated daily event-based surveillance conducted by Field Epidemiology Trainee Program participants from six Member States; participants reported of over 500 cases of animal and human avian influenza A virus infections including subtypes H5N1, H5N2, H5N3, H5N6, H5N8, H6N1, H7N2, H7N9, H9N2, and H10N8.
- Facilitated event information site reports for 318 human cases of avian influenza A (H7N9) virus infection, three human cases of avian influenza A (H10N8) and two human cases of avian influenza A(H9N2).
- Participated in the Cambodia-Viet Nam Bilateral Meeting on influenza A (H5N1) virus in March 2014, the "Expert Advisory Group for Burden of Influenza," in Geneva, December 2013, and the "FAO/WHO sub-regional technical consultation to review preparedness, surveillance and response for Avian Influenza A(H7N9) in Lao PDR, Myanmar, and Vietnam," in Yangon, Myanmar, April 2014.
2015
- Developed an action plan for improved reporting and utilization of influenza surveillance data.
- Developed a prototype for interactive web-based influenza reporting to display both epidemiological (FluID) and virological (FluNet) data.
- Published biweekly regional situational updates of seasonal influenza, weekly updates of avian influenza, and the online Western Pacific Surveillance and Response Journal (WPSAR).
- Supported the Surveillance Officer, Monitoring and Evaluation Technical Officer, Coordinating Editor of WPSAR, Risk Communications Officer, and administrative assistants.
Cambodia
2014
- Investigated 17 laboratory-confirmed human infections of avian influenza A(H5N1) virus, notified WHO through the IHR mechanism and shared information with partners.
- Supported the Communicable Disease Control (CDC) Department and Provincial Health Department to investigate an influenza outbreak in a prison in Battambang Province.
- Developed and trained on a joint pilot protocol between human and animal health sectors on outbreak investigations of avian influenza in Kampot and Tekeo provinces.
2015
- Conducted 12 small outbreak investigations in poultry.
- Maintained eight SARI and seven ILI sites.
- Supported a suspected outbreak of influenza in Pursat Province.
- Supported national and provincial teams with biannual quality assurance visits to ILI sentinel surveillance sites.
- Provided refresher trainings for ILI surveillance staff as well as trainings to enhance information sharing among sentinel sites.
- Supported the Cambodia Early Warning and Alert Network (CamEWARN) indicator-based surveillance system.
- Issued Monthly Respiratory Bulletins in collaboration with CDC Department, MoH, AFRIMS, NAMRU and Institut Pasteur du Cambodge and shared it with partners
- Supported the Team Leader, National Professional Officer, and the National Professional Officer for Surveillance as well as the administrative support group in Emerging Disease Surveillance and Response for influenza surveillance, outbreak investigations, and infection control.
Lao People’s Democratic Republic (PDR)
2014
- Introduced new SARI case definitions and forms to hospital directors, deputies, coordinators, and ILI/SARI sentinel surveillance staff.
- Conducted the Annual Surveillance and Response Workshop in March 2014 to review past activities, outbreaks, and to discuss gaps in surveillance systems.
- Conducted the Annual Lao FET Alumni Conference to share outbreak investigation experiences with new trainees.
2015
- Conducted 18 outbreak investigations, two of which were in persons with ILI.
- Conducted a joint human-animal health meeting on avian influenza surveillance and response with staff and stakeholders.
- Maintained surveillance at eight ILI and five SARI sentinel sites at 13 provincial hospitals.
- Supported the upgrade of the Ministry of Health LaoEWARN surveillance informatics infrastructure.
- Supported ILI and SARI sentinel surveillance in five provinces, with technical assistance for seasonal influenza surveillance, conducting workshops and providing regular site monitoring.
- Distributed weekly laboratory results to all sentinel surveillance sites.
- Carried out joint field supervision in collaboration with NCLE and US CDC.
- Provided support for an Epidemiologist, National Professional Officer for FETP, Laboratory Specialist, and an administrative assistant.
Pacific Island Countries and Territories (PICT)
2014
- Developed site assessment checklists for laboratory and ILI surveillance.
- Revised standard operating procedures (SOP) for ILI surveillance and sample transport outside of Suva.
- Developed national SARI surveillance guidelines.
- Developed posters and promotional material to be used at ILI and laboratory surveillance sites.
- Revised surveillance reporting formats for the Influenza Technical Working Group (TWG), the weekly surveillance report and the monthly Fiji Surveillance Bulletin.
- Registered with FluNet to allow entry of PICT data.
- Initiated SARI surveillance in the intensive care unit of the Commonwealth War Memorial Hospital in Suva, Fiji.
- Provided support to the sub-regional NIC (Fiji CDC - Mataika House).
- Developed proposal for the implementation of event-based surveillance in Pacific settings.
2015
- Integrated influenza surveillance into the Ministry of Health National Plan.
- Produced the first influenza bulletin integrating syndromic and virologic data.
- Supported the Early Warning and Response System (EWARS) to monitor potential communicable disease outbreaks after Cyclone Pam in Vanuatu.
- Provided technical support on surveillance in Papua New Guinea (PNG).
- Supported an outbreak investigation in the Federal States of Micronesia.
- Coordinated influenza surveillance through the National Influenza Surveillance TWG comprised of WHO and MOH representatives.
- Provided assistance for both laboratory and syndromic (ILI/SARI) surveillance to the ministries of health by supporting influenza surveillance officers for Fiji, Solomon Islands, and Vanuatu.
- Supported a Medical Officer (Team Leader), Surveillance Coordinators (Vanuatu, Fiji, Solomon Islands), and a Laboratory Specialist for the Fiji NIC.
Laboratory
Support was provided for the procurement of reagents and supplies, specimen transport and testing. Procurement needs were determined based on influenza External Quality Assessment scores. Technical support was also provided at the laboratory sites to ensure proper testing methods, sample handling and biosafety protocols were followed. Technical support was provided to improve virological data reporting through the GISRS FluNet system. Support was also provided to national laboratories to process samples for outbreak investigations as well as routine surveillance activities.
Laboratory Activities
WPRO
- Provided technical guidance and advice to laboratories in member states on the referral of specimens between laboratories.
- Helped select laboratories and NICs obtain equipment, including a -80°C freezer for PNG. Laboratories also received reagents and disposables for ensuring clean and safe laboratory work.
- Worked closely with WHO CCs, NICs, and other relevant laboratories to plan for the 9th meeting of NICs and Influenza Surveillance in Cambodia (August 2015).
Cambodia
- Supported the procurement of reagents and supplies, shipping, and testing of SARI and ILI samples at the National Institute of Public Health (NIPH). In 2015, NIPH tested approximately 300 SARI and 390 ILI samples.
- Supported confirmatory testing by the NIPH on all positive samples and 10% of all negative samples. NIPH tested 66 event-based surveillance specimens from SARI cases for influenza A (H5N1) and 35 SARI specimens for influenza A (H7N9) viruses.
- Supported weekly transportation of specimens from seven provincial sentinel sites to Phnom Penh for testing.
Lao People’s Democratic Republic (PDR)
- Procured influenza laboratory supplies for the National Centre for Laboratory and Epidemiology (NCLE) in Vientiane including reagents for detecting avian influenza A (H7N9) and repaired critical laboratory equipment.
- Tested an average of 53 outbreak and surveillance specimens for influenza viruses each week in 2014 and found the influenza positivity proportion to range from 0 to 64%.
- Shared 13 samples from persons suspected of having avian influenza with the National Institute of Infectious Diseases (NIID) in Japan; all samples were negative for influenza viruses.
- Tested approximately 70 specimens from outbreaks and surveillance for influenza viruses each week during the first quarter of 2015.
- Sent 22 influenza isolates and three clinical samples to U.S. CDC, and 20 influenza insolates to NIID in November 2014.
- Sent 21 influenza isolates and three samples to U.S. CDC and 20 isolates to NIID in January 2015.
- Conducted field monitoring and on-the-job training in specimen collection and biosafety at all sentinel and non-sentinel sites in 17 provinces.
- Supported a Laboratory Specialist.
Pacific Island Countries and Territories (PICT)
- Resumed contribution of virological data to FluNet.
- Tested samples from ILI sites and from SARI site at CWM Hospital.
- Worked with WHO CC in Melbourne for sequencing and further testing of influenza positive samples.
- Validated new influenza diagnostic reagents.
- Introduced and validated the influenza A H7 RT-PCR assay at Fiji NIC.
- Revised standard operating procedures.
- Received primers and probes for MERS-CoV testing.
Preparedness
Capacities of IHR National Focal Points (NFPs) were strengthened and tested through an IHR communication exercise called “Crystal” in December 2013 and 2014. PanStop was carried out in February 2014, to practice, validate, and strengthen procedures for determining if a rapid containment operation is necessary to stop or slow the spread of an outbreak of influenza with pandemic potential.
In 2015, there was a focus on the integration of the animal and human sectors for preparedness activities. Participants from the Western Pacific countries participated in a multi-sectoral workshop which focused on zoonotic influenza viruses. Standard operating procedures were also developed for outbreak and emergency response. Although the PanStop exercise could not be conducted in 2014 due to large scale deployment to support the Ebola outbreak, the experience gained through Ebola response and the lessons learned for effective response will be important for future public health emergencies.
Other support for preparedness included activities for Field Epidemiology Training fellows, procurement of supplies for rapid response teams, and human resources for technical guidance on outbreak response and for health authorities developing, delivering and evaluating training programs.
Preparedness Activities
WPRO
- Conducted the fifth annual IHR exercise (IHR Exercise Crystal) to test the capacity for and adherence to communications requirements outlined in IHR (2005) (December 2013).
- Conducted an additional IHR communication exercise (December 2014).
- Conducted a rapid containment exercise called PanStop in Manila (February 2014).
- Designated the WPRO Influenza Technical Officer to participate in the 3rd WHO Informal Consultation on Improving Influenza Vaccine Virus Selection (April 2014).
- Enabled IHR National Focal Points to test their preparedness and response procedures and highlighted the application of pandemic preparedness plans to strengthen all-hazards public health emergency planning through a Regional Ebola Simulation Exercise.
- Supported participation of Western Pacific countries in the Fifth Asia Pacific Workshop on Multi-Sectoral Collaboration for the Prevention and Control of Zoonosis (November 2014).
- Supported the annual testing of member states’ capacity for and adherence to communications requirements outlined in IHR.
- Participated in monthly risk assessments and held monthly three-level (Country Office, Regional Office, Headquarters) and tripartite (FAO, OIE, WHO) teleconferences in response to the second (2013/2014) and third (2014/2015) waves of human infection of avian influenza A(H7H9) virus in China.
Cambodia
- Provided technical support to develop outbreak investigation SOPs that address multi-sectoral coordination, deployment of personnel during an outbreak, procurement, transport, and risk communication.
- Supported World Hand Hygiene Day 2014.
- Supported 2014 Infection Prevention Workshop.
- Provided technical support to revise training materials for clinicians on clinical management for severe acute respiratory infection (SARI).
- Conducted an assessment of the Institut Pasteur du Cambodge (IPC) capacity and isolation units of five hospitals.
Lao People’s Democratic Republic (PDR)
- Supported a public health emergency preparedness simulation exercise with approximately 80 national rapid response training (RRT) participants.
- Supported an epidemiologist for influenza preparedness and outbreak response activities.
Pacific Island Countries and Territories (PICT)
- Commenced planning for review of Fiji Pandemic Preparedness and Response Plan.
- Developed an event-based surveillance proposal for implementation in Pacific island countries.
- Commenced planning for Fiji National Influenza Surveillance Meeting.
- Reviewed and updated personal protective equipment (PPE) stockpile.
Training
WPRO
- Adapted WHO manual for estimating burden of influenza disease for application in selected countries of the Western Pacific Region.
- Provided technical support for a two-day Infectious Substances Shipping Training (ISST) in Fiji for 29 participants.
Cambodia
- Supported a National Epidemiology Conference in September 2014.
- Participated in ILI surveillance workshops for information sharing and refresher training conducted by the Ministry of Health (MOH).
- Conducted Applied Epidemiology Training (AET) on influenza surveillance and IHR core capacities.
- Supported an AET introductory course on outbreak investigation and response.
- Supported AET field activities including investigation of ILI clusters and human infections with avian influenza.
- Drafted an infection safety training curriculum for healthcare workers, including an assessment and planning tool for infection, prevention and control in isolation rooms.
- Provided technical support for Rapid Response Team training in investigation of human infection with avian influenza, risk assessment, and specimen collection.
- Organized training workshop for teachers from public and private medical/co-medical universities and colleges on infection, prevention and control.
Lao People's Democratic Republic (PDR)
- Provided technical and financial support to the one-year Lao Field Epidemiology Training (FET). FET Cohort V graduated in February 2014.
- Initiated FET Cohort VI which completed Module III lectures, field work, and data collection in March 2014.
- Conducted training on case management of SARI at one sentinel hospital in April 2014.
- Supported six nurses, from six different hospitals, to attend an infection prevention and control course in Thailand, March – June 2015.
Pacific Island Countries and Territories (PICT)
- Trained on infection, prevention and control for influenza in Vanuatu.
- Developed training material for three-day surveillance and outbreak response workshop for Divisional Response Teams in Fiji.
- Developed and provided materials to surveillance sites including a sample collection video, SOPs, and posters.
- Conducted site visits to laboratory-based surveillance sites, providing on-site refresher training.
Contacts
Ailan Li, MD
Director
Emerging Disease Surveillance and Response
World Health Organization
Regional Office for the Western Pacific
Manila, Philippines
Email: lia@wpro.who.int
Chin Kei Lee, MB ChB, MPH, MAE
Coordinator
Emerging Disease Surveillance and Response
World Health Organization
Regional Office for the Western Pacific
Manila, Philippines
Email: leec@wpro.who.int
Erica Dueger, DVM, PhD
Medical Officer
Emerging Disease Surveillance and Response
World Health Organization
Regional Office for the Western Pacific
Manila, Philippines
Email: duegere@wpro.who.int
Jun Nakagawa, PhD
Programme Management Officer
Division of Health Security and Emergencies
World Health Organization
Regional Office for the Western Pacific
Manila, Philippines
Email: nakagawaj@wpro.who.int
Franciscus Konings, PhD
Technical Officer
Emerging Disease Surveillance and Response
World Health Organization
Regional Office for the Western Pacific
Manila, Philippines
Email: koningsf@wpro.who.int
Sarah Hamid, MPH Consultant
Emerging Disease Surveillance and Response
World Health Organization
Regional Office for the Western Pacific
Manila, Philippines
Email: hamids@wpro.who.int
- Page last reviewed: June 14, 2016
- Page last updated: June 14, 2016
- Content source:
Error processing SSI file


 ShareCompartir
ShareCompartir