WHO European Region (EUR) 2014-2015
Currently, there are seven bilateral influenza cooperative agreements that support influenza activity in the European Region. These cooperative agreements are with ministries of health or other institutions that work with the U.S. Centers for Disease Control and Prevention (CDC) to build capacity in order to routinely identify, diagnose and respond to seasonal and pandemic influenza.
Direct Country Support
Direct country support though non-research cooperative agreements is established in the following seven countries/entities:
- Armenia [312 KB, 2 pages]
- Georgia [237 KB, 2 pages]
- Kyrgyzstan [198 KB, 2 pages]
- Republic of Moldova [246 KB, 2 pages]
- Russian Federation [269 KB, 2 pages]
- SECID: The Southeast European Center for Surveillance and Control of Infectious Diseases - (Priority countries - Albania, Bosnia and Herzegovina, Kosovo*, Macedonia, and Montenegro) [141 KB, 3 pages]
- Ukraine [180 KB, 2 pages]
In addition, CDC supports the World Health Organization (WHO) Regional Office for Europe via a cooperative agreement to provide technical and coordination support to Member States.
*This designation is without prejudice to positions on status, and is in line with UNSCR 1244 and the ICJ Opinion on the Kosovo declaration of independence.
Core Activities
The core activities of these bilateral agreements are:
- To build sustainable national capacity for the detection, identification and response to seasonal, avian and novel influenza.
- To develop interagency pandemic preparedness plans.
- To strengthen capacity for integrated laboratory and epidemiologic surveillance for influenza-like illness (ILI) and severe acute respiratory infections (SARI), which includes making routine contributions to WHO’s Global Influenza Surveillance and Response System (GISRS) and implementing International Health Regulations 2005 (IHR).
- To develop and train local rapid response and containment teams.
Influenza Division Contacts
Stacey Spivey-Blackford, MS
Project Officer
Extramural Program
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Atlanta, GA
Email: ifm8@cdc.gov
Mark Thompson, PhD
Health Scientist
International Epidemiology and Research Team
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Atlanta, GA
Email: mthompson2@cdc.gov
WHO Regional Office for Europe (EURO)
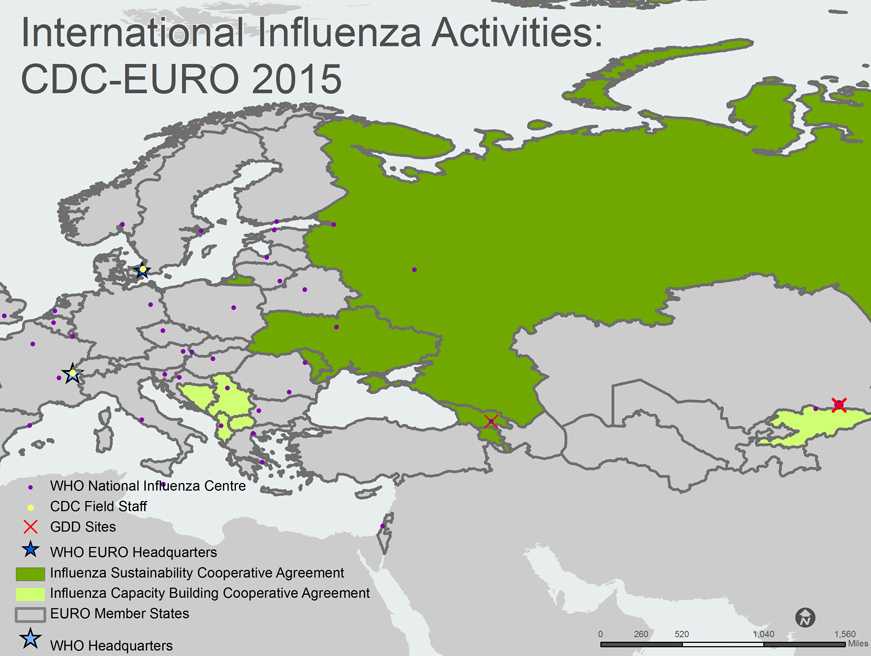
A map of the WHO European Region (EUR) shows all 53 EUR member states/countries. The member countries, outlined with gray borders, include Albania, Andorra, Armenia, Austria, Azerbaijan, Belarus, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Georgia, Germany, Greece, Hungary, Ireland, Israel, Italy, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Luxembourg, Malta, Monaco, Montenegro, Netherlands, Norway, Poland, Portugal, Republic of Moldova, Romania, Russian Federation, San Marino, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Tajikistan, The Former Yugoslav Republic of Macedonia, Turkey, Turkmenistan, Ukraine, United Kingdom of Great Britain and Northern Ireland, and Uzbekistan.
Countries with yellow shading indicate that the Influenza Division provides project funding and technical assistance through Capacity Building Cooperative Agreements. Kyrgyzstan and the Southeast European Center for Surveillance and Control of Infectious Diseases (SECID) priority countries (Albania, Bosnia and Herzegovina, The Former Yugoslav Republic of Macedonia, Montenegro, and Kosovo*) are shaded light green on the map. Armenia, Georgia, Moldova, the Russian Federation, and Ukraine are shaded green to indicate that they have a Sustainability Cooperative Agreement.
CDC Influenza Division Field Staff, indicated by a yellow dot, are located at the WHO Regional Office for Europe in Copenhagen, Denmark and at WHO Headquarters in Geneva, Switzerland.
The Global Disease Detection [GDD] Sites, indicated by the red “X”, are located in Georgia and Kazakhstan.
WHO National Influenza Centers (NICs), indicated by a purple dot, are located in Albania (Tirana), Austria (Vienna), Belarus (Minsk), Belgium (Brussels), Bulgaria (Sofia), Croatia (Zagreb), Czech Republic (Prague), Denmark (Copenhagen), Estonia (Tallinn), Finland (Helsinki), France (Lyon and Paris), Georgia (Tbilisi), Germany (Berlin), Greece (Athens and Thessaloniki), Hungary (Budapest), Iceland (Reykjavik), Ireland (Dublin), Israel (Tel Hashomer), Italy (Rome), Kazakhstan (Almaty), Kyrgyzstan (Kyrgyzstan), Latvia (Riga), Lithuania (Vilnius), Luxembourg (Luxembourg), Malta (Msida), Netherlands (Rotterdam), Norway (Oslo), Poland (Warsaw), Portugal (Lisboa), Romania (Bucharest [2] and Lasi), Russia (Moscow and St. Petersburg), Serbia (Belgrade and Novi Sad), Slovakia (Bratislava), Slovenia (Ljubljana), Spain (Barcelona, Madrid and Valladolid), Sweden (Solna), Switzerland (Geneva), Turkey (Ankara), Ukraine (Kiev), and United Kingdom (Aberdeen, London and Glasgow).
The WHO Regional Office for Europe (EURO), indicated by a blue star, is located in Copenhagen, Denmark. WHO Headquarters is located in Geneva, Switzerland.
Highlights
- Launched the new joint WHO/Europe and European Centre for Disease Prevention and Control (ECDC) influenza surveillance platform and bulletin, Flu News Europe, for 53 countries in October 2014.
- Held the Fourth Joint WHO Regional Office for Europe/ECDC Meeting on Influenza Surveillance (2014).
- Organized a Flu Awareness Campaign in October 2014 with a focus on increasing uptake of seasonal influenza vaccine in high risk groups.
- Deployed two Influenza and Respiratory Pathogens Program (IRP) staff to Sierra Leone and one to Liberia to support the response to Ebola.
- Published 11 articles in international, peer-reviewed journals, including a multicountry SARI risk factor study [832 KB, 12 pages].
- Updated and translated into Russian the WHO Regional Office for Europe Influenza website.
U.S. CDC Direct Support
The WHO Regional Office for Europe (WHO/Europe) in Copenhagen, Denmark, serves 53 Member States with a population exceeding 900,000 million. Influenza activities are conducted by the Influenza and Other Respiratory Pathogens Programme (IRP).
The second five-year cooperative agreement (CoAg) began in September 2011 and entered its fourth year in October 2014. In addition to financial support, since 2009 a CDC-seconded senior epidemiologist has strengthened IRP. CoAg activities are grouped around the following technical areas: surveillance and laboratory; seasonal influenza vaccine; burden of disease; pandemic preparedness and early warning; and communication and advocacy. IRP also collaborates with CDC staff on the development of trainings (e.g. data management training) and tools (e.g. influenza surveillance assessment tool).
Coordinating influenza surveillance and providing support to countries in the WHO European Region is a major activity of the CoAg. Until October 2014, IRP ran the regional influenza surveillance platform and published the weekly surveillance bulletin, EuroFlu, in English and Russian.
WHO/Europe also continues to provide training and technical assistance to member states to strengthen influenza surveillance. In addition, WHO/Europe supports National Influenza Centres (NIC) throughout the region by providing external quality assessment (EQA) programs, and by supporting virus strain characterization, and sharing of influenza viruses within the Global Influenza Surveillance and Response System (GISRS).
Surveillance
The WHO Regional Office for Europe strives to strengthen epidemiological and virological components of sentinel surveillance for influenza, including outpatient surveillance for influenza-like illness (ILI) and acute respiratory infection (ARI), and hospital-based surveillance for severe acute respiratory infections (SARI). The activities in this area include collecting, analyzing and publishing in Flu News Europe weekly surveillance data from 50 countries; developing capacity to use surveillance data to estimate the burden of influenza to prioritize national influenza vaccination programmes; increasing uptake of seasonal influenza vaccine; and supporting activities at the national level aimed at implementing International Health Regulations core capacities for early warning and response. Countries of the Newly Independent States, where sentinel surveillance has recently been established, and selected countries of South-Eastern Europe (SEE) continued to be the main focus of the work at the country level from 2013 to 2015.
Surveillance Activities
- Continued development of epidemic thresholds for SARI surveillance based on the Moving Epidemic Method.
- Supported strengthening of influenza surveillance in the region, including assistance to SEE countries through a CoAg with CDC.
- Expanded the Flu Awareness Campaign, a multimedia event, with six member states participating in the 2014–2015 season.
- Developed tools to support reviewing, monitoring and strengthening national surveillance systems, including an electronic surveillance assessment tool and a feasibility tool for select SARI sentinel sites.
- Enhanced disease surveillance for severe influenza in the region, with 15 countries routinely conducting SARI surveillance by 2015.
- Conducted inter-country meetings and missions to three countries to support calculation of estimates of clinical and economic influenza burden.
- Continued the development of guidelines to increase influenza vaccine uptake in targeted populations (i.e. pregnant women and health care workers) based on the Tailoring Immunization Programmes for Influenza.
Laboratory
In the European Region, 41 (77%) of 53 countries with influenza surveillance have a National Influenza Centre (NIC) recognized by WHO. Through the CoAg, NICs in the WHO European Region receive training in influenza laboratory techniques, support to improve laboratory quality, assistance with shipment of viruses to WHO Collaborating Centres for reference and research on influenza, and reagents for influenza testing. A total of 44 (83%) countries in the WHO European Region share influenza viruses with GISRS, and 16 (30%) monitor and report weekly data on antiviral susceptibility to WHO.
Laboratory Activities
- Increased the number of laboratories in the region participating in the WHO External Quality Assurance Programme from 34 in 29 countries in 2007 to 63 in 48 countries in 2014.
- Provided support for 28 countries to ship viruses and clinical specimens to the WHOCC in time for the WHO Consultation on the Composition of Influenza Virus Vaccines (VCM) for the Northern Hemisphere 2015–2016 influenza season. Of these, 16 countries used the WHO Shipment Fund Project.
- Provided three national trainings on shipping infectious substances; participants were 60 specialists from national, regional, and sub-regional levels and reference laboratories in Tajikistan, Turkmenistan, and Uzbekistan.
- Conducted a training course for 17 virologists from the European Region on laboratory preparedness for emerging respiratory pathogens.
- Organized and held the training “Introduction to Laboratory Quality Management and the Laboratory Quality Stepwise Implementation (LQSI) tool” for all SEE countries and for all Newly Independent States.
- Organized the WHO course “Strengthening capacities of influenza laboratory experts” for NICs.
Preparedness
In the period from October 1, 2013 to September 30, 2015 several outbreaks highlighted the continued importance of pandemic preparedness. The main events during the last two years have been the ongoing outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) in the Arabian Peninsula, which exported cases to the WHO European Region; human cases of avian influenza A(H7N9) virus infection in China, a country which borders the WHO European Region; a substantial rise in the number of human cases of avian influenza A(H5N1) virus infection in Egypt; and the largest ever outbreak of Ebola virus in Western Africa, including a number of cases imported to Europe.
These outbreaks with their complexities and challenges emphasized the need for WHO and its member states to continue to strengthen core capacities of the International Health Regulations and pandemic preparedness.
Preparedness Activities
- Held critical care training for a total of 140 intensive care clinicians in five countries.
- Held a workshop on outbreak response to avian influenza A(H7N9) virus infections and other emerging pathogens in three countries (Kyrgyzstan, Tajikistan, and Turkmenistan).
- Held a Joint ECDC and WHO/Europe Consultation on pandemic and all hazard preparedness in November 2013.
- Switzerland and Germany published a revised pandemic preparedness plan, which brings the total to eight countries having published revised pandemic preparedness plans since the 2009 pandemic.
- Reorganized the WHO/Europe web site on pandemic influenza and translated it into Russian.
- Conducted laboratory preparedness surveys for avian influenza A(H7N9) virus and MERS-CoV.
- Provided technical assistance in the European Region and West Africa during outbreaks caused by MERS-CoV, avian influenza virus, and Ebola virus.
- Conducted a workshop on outbreak investigation and response for South-eastern European countries in collaboration with the South East European Center of Infectious Diseases Surveillance and Control (SECID) in July 2015.
Training
- Conducted a joint ECDC and WHO/Europe consultation on pandemic and all-hazard preparedness in Slovakia (November 2013).
- Conducted Data Management Training for South East European (SEE) Countries in collaboration with CDC in Greece (April 2014).
- Conducted the 4th joint WHO/Europe–ECDC Annual European Influenza Surveillance Meeting in Austria (June 2014).
- Conducted a training on defining disease burden and decision-making for seasonal influenza vaccination for eight countries in Georgia (August 2014).
- Developed the Introduction to Laboratory Quality Management and the Laboratory Quality Stepwise Implementation (LQSI) tool for SEE and NIS, (November 2014/April 2015).
- Conducted a laboratory preparedness training course in the Netherlands (November 2014).
- Conducted a course for NICs on development and validation of PCR assays in the Russian Federation (May 2015).
- Conducted a workshop to estimate disease burden for seasonal influenza for four countries from SEE in Denmark (July 2015).
Contacts
Caroline Brown, PhD
Programme Manager
Influenza & Other Respiratory Pathogens Programme WHO Regional Office for Europe
World Health Organization
Copenhagen, Denmark
Email: cbr@euro.who.int
Diane Gross, DVM, PhD
Epidemiologist
Influenza & Other Respiratory Pathogens Programme WHO Regional Office for Europe
World Health Organization
Copenhagen, Denmark
Email: dgo@euro.who.int
Michala Hegermann-Lindencrone, MPH
Technical Officer
Influenza & Other Respiratory Pathogens Programme WHO Regional Office for Europe
World Health Organization
Copenhagen, Denmark
Email: mhl@euro.who.int
- Page last reviewed: June 14, 2016
- Page last updated: June 14, 2016
- Content source:
Error processing SSI file


 ShareCompartir
ShareCompartir