WHO South-East Asia Region (SEAR) 2014-2015
In fiscal year 2014, the U.S. Centers for Disease Control and Prevention (CDC) funded nine non-research bilateral influenza cooperative agreements in the South-East Asia Region (SEAR). Cooperative agreements with ministries of health (MOH) or institutions designated by the MOH build capacity to routinely identify, diagnose and respond to seasonal, avian and pandemic influenza.
Direct Country Support
Direct country support through cooperative agreements is established in the following eight countries:
- Bangladesh [336 KB, 3 pages]
- Bhutan [196 KB, 3 pages]
- India (until Sept 2014) [134 KB, 2 pages]
- Indonesia [135 KB, 2 pages]
- Maldives [275 KB, 2 pages]
- Nepal [152 KB, 2 pages]
- Sri Lanka [272 KB, 2 pages]
- Thailand (until Sept 2014) [177 KB, 2 pages]
In addition, CDC supports the World Health Organization (WHO) Regional Office for South-East Asia (SEARO) Headquarters in New Delhi through a cooperative agreement.
In 2014, three countries (India, Indonesia and Thailand) entered the final year of the five-year cooperative agreement designed to transition to local sustainability. Bangladesh entered the fourth year of their sustainability agreement while Nepal and Sri Lanka entered the second year.
Maldives and Bhutan entered the second year of the capacity building grant cycle.
Countries are expected to develop and maintain a surveillance system that rapidly detects, identifies and responds to seasonal, novel and pandemic influenza, participate in WHO’s Global Influenza Surveillance and Response System (GISRS) and create and implement a sustainability plan that phases out U.S. government funding. All but two countries have a laboratory designated as a WHO National Influenza Centers (NIC), and the two that do not are working toward designation for their national public health laboratories.
Core Activities
Core activities include improving laboratory and epidemiologic capacity and infrastructure for influenza virologic and disease surveillance; developing hospital-based sentinel surveillance for influenza-like illness (ILI) and severe acute respiratory infections (SARI); integrating laboratory and epidemiologic influenza surveillance; developing and maintaining surveillance for cases and clusters of respiratory illnesses; and training local rapid response and containment teams.
Influenza Division Contacts
Karen Siener, MPH
Public Health Advisor
Extramural Program
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Atlanta, GA
Email: khs3@cdc.gov
Danielle Iuliano, MPH, PhD
Senior Research Scientist
International Epidemiology and Research Team
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Atlanta, GA
Email: aoi0@cdc.gov
Fatimah Dawood, MD
Medical Officer/Epidemiologist
International Epidemiology and Research Team
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Atlanta, GA
Email: hgj0@cdc.gov
Katie Lafond, MPH
Epidemiologist
International Epidemiology and Research Team
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Atlanta, GA
Email: gmj3@cdc.gov
Katharine Sturm-Ramirez, PhD
CDC Influenza Program Director, Bangladesh
U.S. Embassy
Dhaka, Bangladesh
Email: kgi4@cdc.gov
Kim Lindblade, PhD, MPH
CDC Influenza Program Director, Thailand
Thailand MOPH/U.S. CDC Collaboration
Bangkok, Thailand
Email: kil2@cdc.gov
Seema Jain, MD (as of August 2015)
CDC Influenza Program Director, India
U.S. Embassy
New Delhi, India
Email: bwc8@cdc.gov
WHO Regional Office for South-East Asia (SEARO)
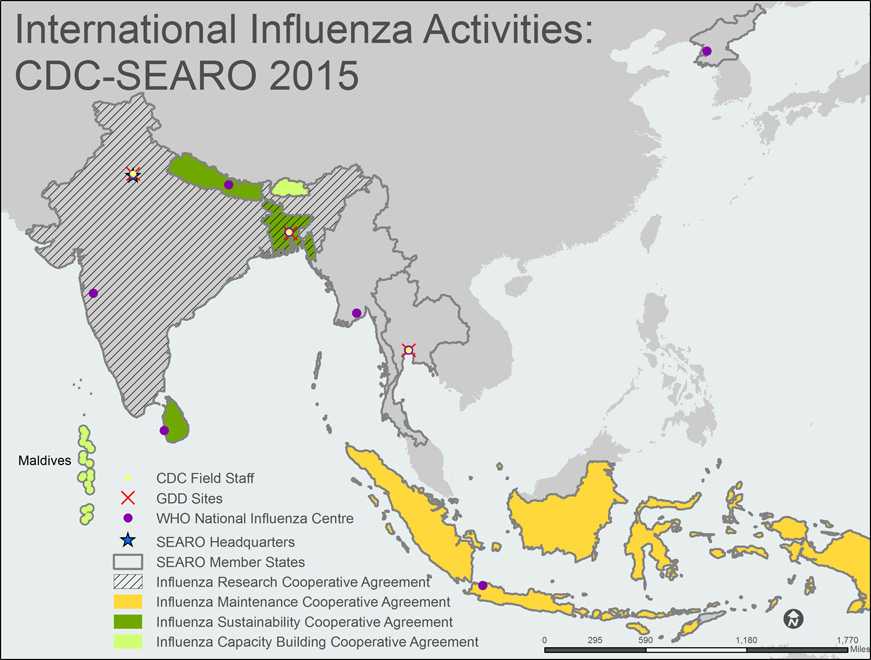
A map of the WHO South-East Asia Region (SEAR) shows all 11 SEAR member states/countries. The member countries, outlined with gray borders, include Bangladesh, India, Indonesia, Nepal, Sri Lanka, Thailand, Bhutan, DPR Korea, Myanmar, Maldives and Timor-Leste.
Countries with shading indicate that the Influenza Division provides project funding and technical assistance through cooperative agreements. Bhutan and the Maldives are shaded light green to indicate Capacity Building Cooperative Agreements. Bangladesh, Nepal, and Sri Lanka are shaded green to indicate Sustainability Cooperative Agreements. Bangladesh and India also have black diagonal stripes across the country to indicate Research Cooperative Agreements. Indonesia is shaded yellow to indicate a Maintenance Cooperative Agreement.
CDC Field Staff, indicated by a yellow dot, are located in the following cities: Bangkok, Dhaka and New Delhi.
The Global Disease Detection [GDD] Sites, indicated by red X’s, are located in Bangkok, Thailand; Dhaka, Bangladesh; and New Delhi, India.
WHO National Influenza Centers (NICs), indicated by a purple dot, are located in the following cities: Colombo, Dhaka, Jakarta, Kathmandu, Nonthaburi, Pune, Pyongyang, and Yangon.
The WHO Regional Office for South-East Asia (SEARO), indicated by a blue star, is located in New Delhi, India.
Highlights
- Organized the 8th Bi-Regional Influenza Surveillance and NIC Meeting in Jakarta, Indonesia.
- Developed a methodology to assess the cost-effectiveness of seasonal influenza vaccination in Nepal.
- Conducted a systematic analysis of influenza disease and economic burden and cost-effectiveness of influenza vaccines in the South-East Asia region.
- Established the capacity of clinicians to manage patients with avian influenza and other severe respiratory infections.
U.S. CDC Direct Support
Since 2006, WHO’s Regional Office for South-East Asia (SEARO) has received funding from a CDC cooperative agreement to support enhancing the capacity of member states to build and maintain an influenza surveillance system which is used for the routine identification, investigation, and containment of novel influenza viruses, some of which may have pandemic potential.
WHO SEARO is located in New Delhi, India. The office serves the following 11 member countries: Bangladesh, Bhutan, DPR Korea, India, Indonesia, Maldives, Myanmar, Nepal, Sri Lanka, Thailand and Timor-Leste. Eight of the 11 countries received cooperative agreement funds from CDC’s Influenza Division in fiscal year 2014.
In 2013–2014, WHO SEARO staff provided training, support and technical assistance to member countries to strengthen preparedness and response, surveillance and laboratory capacity. Working together, staff working on the International Health Regulations (IHR), Immunization and Vaccine Development (IVD) and Health Laboratory Systems (HLS) together implemented the activities related to the CDC grant.
In the coming year, WHO SEARO will focus on further strengthening capacity development in disease surveillance, preparedness for and response to seasonal and pandemic influenza, and scaling-up laboratory capacity in influenza diagnostics.
Surveillance
SEARO has utilized both CDC and WHO pandemic influenza preparedness (PIP) funds to conduct trainings and laboratory activities to improve influenza surveillance in the region.
Surveillance Activities
- Facilitated collating and synthesizing available regional and country-level data on economic and disease burden of influenza.
- Facilitated a discussion with Sri Lanka and Bhutan to improve reporting of influenza surveillance data to WHO FluID.
- Supported member countries by planning and implementing a number of activities to improve surveillance at the country level.
Laboratory
The financial support provided to SEARO through the cooperative agreement was used to enhance the capacity of the influenza laboratories in the region by conducting a regional laboratory workshop on diagnosis of influenza and novel respiratory viruses. This training was held at the national Institute of Virology in Prune, India from May 26-30, 2014. The main objective of the workshop was to provide hands-on training on using diagnostic tools including RT-PCR for influenza and novel respiratory viruses. In addition, the cooperative agreement supported the participation of laboratory focal points from the NICs and public health laboratories in the 7th and 8th NIC meetings in 2013-2014.
Laboratory Activities
WHO SEARO
- Conducted a regional laboratory workshop on diagnosis of influenza and novel respiratory viruses.
- Assessed two influenza public health laboratories and provided on-site trainings to strengthen laboratory capacity including molecular diagnosis.
Bhutan
- Assigned a consultant to review the standard operating procedures for influenza RT-PCR and influenza RT-PCR quality systems; developed bio-safety guidelines; and trained the influenza laboratory staff on influenza RT-PCR procedures.
Timor-Leste
- Trained staff in molecular diagnostics and line probe assay testing for avian influenza A (H7N9) virus; developed new and updated existing standard operating procedures; developed molecular diagnostic modules and modules on material safety datasheets for all influenza-specific reagents used in PCR; trained staff using these modules and orientated staff on specimen repository, management and shipping.
Preparedness
SEARO identified the need to review existing member countries’ pandemic influenza preparedness and response plans, including pandemic vaccine deployment plans to better prepare for and respond to a future pandemic. SEARO recognized the need for familiarizing member countries with the global guidelines on pandemic influenza preparedness and pandemic vaccine deployment.
To facilitate implementation of the above objectives, SEARO held a regional meeting with participation of all member states.
Preparedness Activities
- Organized a regional workshop to build the capacity of non-vaccine producing countries. Discussions included how to register and evaluate commercially-available seasonal and pandemic influenza vaccine.
- Conducted regional training for influenza vaccine manufacturing countries in SEAR that focused on designing, conducting and reviewing studies in support of initial vaccine approval, annual strain change, process modification and prequalification.
- Focused attention on strengthening the regional clinical network, enhancing the clinical management capacity of influenza and other respiratory pathogens of outbreak potential, and facilitating linkages between curative health care and public health services.
- Worked jointly with WHO Country Offices and key stakeholders in six countries to review tabletop exercises—key components of the International Health Regulations (2005)—related to responding to a pandemic or an emerging infectious disease.
Training
- Conducted a training workshop on clinical management of avian influenza and other causes of severe acute respiratory infections (SARI) in Jakarta, Indonesia.
- Conducted a regional workshop on planning for influenza pandemic preparedness in Kathmandu, Nepal.
- Conducted a workshop on sensitizing the influenza vaccine manufacturers in the South-East Asia Region to the Pandemic Influenza Preparedness (PIP) framework.
- Conducted a regional laboratory workshop on influenza and novel respiratory viruses in Pune, India.
- Conducted on-site trainings for laboratory staff at the public health laboratories in Bhutan and Timor-Leste.
Influenza Vaccine Activities
- Supported countries in developing a road map to plan for influenza vaccination using recommendations of the SEAR Immunization Technical Advisory Group (ITAG).
- Focused on strengthening the national regulatory authorities in both influenza vaccine manufacturing and non-manufacturing countries in SEAR to enable timely and rapid deployment of influenza vaccines.
Contacts
Arun Thapa, MBBS, MS
Director, Family, Gender and Life Course
South-East Asia Regional Office
World Health Organization
New Delhi, India
Email: thapaa@who.int
Rajesh Bhatia, MBBS, MD, DIM, DIT
Director, Communicable Diseases
South-East Asia Regional Office
World Health Organization
New Delhi, India
Email: bhatiaraj@who.int
Roderico Hood Ofrin, MD, MPH
Regional Coordinator, Emergency and Humanitarian Action
South-East Asia Regional Office
World Health Organization
New Delhi, India
Email: ofrinr@who.int
Nihal Abeysinghe, MBBS, MSC, MD
Acting Coordinator, Immunization and Vaccine Development
South-East Asia Regional Office
World Health Organization
New Delhi, India
Email: abeysinghen@who.int
Aparna Singh Shah, MBBS, MD
Regional Advisor, Health Laboratory System
South-East Asia Regional Office
World Health Organization
New Delhi, India
Email: shahap@who.int
Pushpa Ranjan Wijesinghe, MD-Med, MPH, MSC, MD-Com Med
Medical Officer, Emerging Vaccine Preventable Disease Surveillance
South-East Asia Regional Office
World Health Organization
New Delhi, India
Email: wijesinghep@who.int
Philip Gould, MD, MPH, FAAFP
Medical Officer, Influenza and other Emerging Pathogen Surveillance
South-East Asia Regional Office
World Health Organization
New Delhi, India
Email: gouldp@who.int
Bardan Rana, MD
Acting Regional Advisor, International Health Regulation
South-East Asia Regional Office
World Health Organization
New Delhi, India
Email: ranab@who.int
- Page last reviewed: June 14, 2016
- Page last updated: June 14, 2016
- Content source:
Error processing SSI file


 ShareCompartir
ShareCompartir