
Amebiasis
[Entamoeba histolytica]
Causal Agents
Several protozoan species in the genus Entamoeba colonize humans, but not all of them are associated with disease. Entamoeba histolytica is well recognized as a pathogenic ameba, associated with intestinal and extraintestinal infections. The other species are important because they may be confused with E. histolytica in diagnostic investigations.
Life Cycle
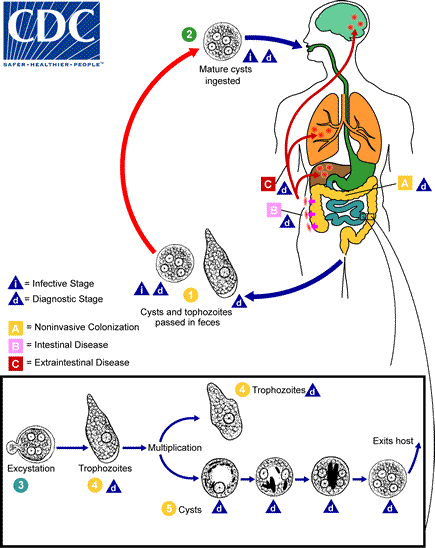
Cysts and trophozoites are passed in feces  . Cysts are typically found in formed stool, whereas trophozoites are typically found in diarrheal stool. Infection by Entamoeba histolytica occurs by ingestion of mature cysts
. Cysts are typically found in formed stool, whereas trophozoites are typically found in diarrheal stool. Infection by Entamoeba histolytica occurs by ingestion of mature cysts  in fecally contaminated food, water, or hands. Excystation
in fecally contaminated food, water, or hands. Excystation  occurs in the small intestine and trophozoites
occurs in the small intestine and trophozoites  are released, which migrate to the large intestine. The trophozoites multiply by binary fission and produce cysts
are released, which migrate to the large intestine. The trophozoites multiply by binary fission and produce cysts  , and both stages are passed in the feces
, and both stages are passed in the feces  . Because of the protection conferred by their walls, the cysts can survive days to weeks in the external environment and are responsible for transmission. Trophozoites passed in the stool are rapidly destroyed once outside the body, and if ingested would not survive exposure to the gastric environment. In many cases, the trophozoites remain confined to the intestinal lumen (
. Because of the protection conferred by their walls, the cysts can survive days to weeks in the external environment and are responsible for transmission. Trophozoites passed in the stool are rapidly destroyed once outside the body, and if ingested would not survive exposure to the gastric environment. In many cases, the trophozoites remain confined to the intestinal lumen ( : noninvasive infection) of individuals who are asymptomatic carriers, passing cysts in their stool. In some patients the trophozoites invade the intestinal mucosa (
: noninvasive infection) of individuals who are asymptomatic carriers, passing cysts in their stool. In some patients the trophozoites invade the intestinal mucosa ( : intestinal disease), or, through the bloodstream, extraintestinal sites such as the liver, brain, and lungs (
: intestinal disease), or, through the bloodstream, extraintestinal sites such as the liver, brain, and lungs ( : extraintestinal disease), with resultant pathologic manifestations. It has been established that the invasive and noninvasive forms represent two separate species, respectively E. histolytica and E. dispar. These two species are morphologically indistinguishable unless E. histolytica is observed with ingested red blood cells (erythrophagocystosis). Transmission can also occur through exposure to fecal matter during sexual contact (in which case not only cysts, but also trophozoites could prove infective).
: extraintestinal disease), with resultant pathologic manifestations. It has been established that the invasive and noninvasive forms represent two separate species, respectively E. histolytica and E. dispar. These two species are morphologically indistinguishable unless E. histolytica is observed with ingested red blood cells (erythrophagocystosis). Transmission can also occur through exposure to fecal matter during sexual contact (in which case not only cysts, but also trophozoites could prove infective).
Geographic Distribution
Worldwide, with higher incidence of amebiasis in developing countries. In industrialized countries, risk groups include male homosexuals, travelers and recent immigrants, and institutionalized populations.
Clinical Presentation
A wide spectrum, from asymptomatic infection ("luminal amebiasis"), to invasive intestinal amebiasis (dysentery, colitis, appendicitis, toxic megacolon, amebomas), to invasive extraintestinal amebiasis (liver abscess, peritonitis, pleuropulmonary abscess, cutaneous and genital amebic lesions).
E. histolytica/E. dispar cysts in concentrated wet mounts.
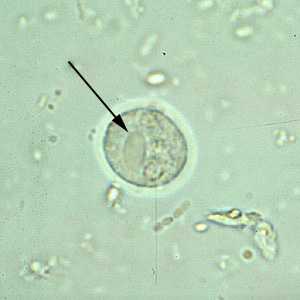
Figure A: Cyst of E. histolytica/E. dispar in an unstained concentrated wet mount of stool. Notice the chromatoid bodies with blunt, rounded ends (arrow).
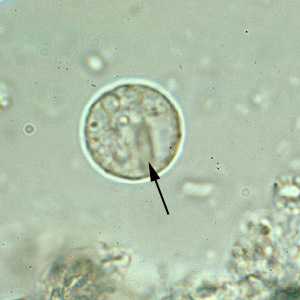
Figure B: Cyst of E. histolytica/E. dispar in an unstained concentrated wet mount of stool. Notice the chromatoid bodies with blunt, rounded ends (arrow).
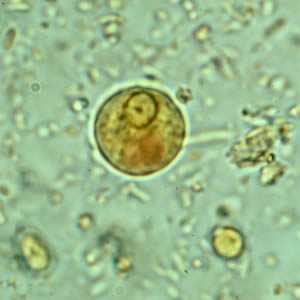
Figure C: Cyst of E. histolytica/E. dispar in a concentrated wet mount stained with iodine.
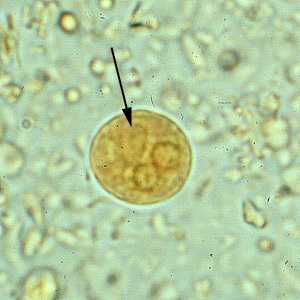
Figure D: Cyst of E. histolytica/E. dispar in a concentrated wet mount stained with iodine. Notice the chromatoid body with blunt, rounded ends (arrow).
E. histolytica/E. dispar cysts stained with trichrome.
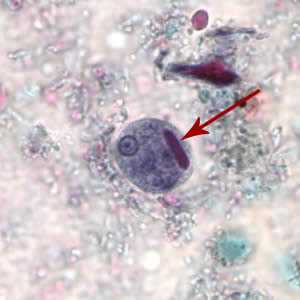
Figure A: Cyst of E. histolytica/E. dispar stained with trichrome. Note the chromatoid body with blunt ends (red arrow).
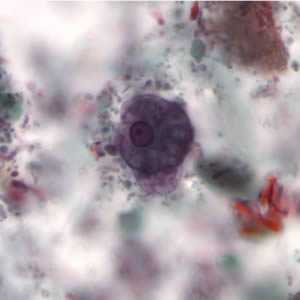
Figure B: Cyst of E. histolytica/E. dispar stained with trichrome.
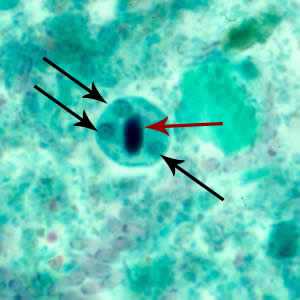
Figure C: Cyst of E. histolytica/E. dispar stained with trichrome. Three nuclei are visible in the focal plane (black arrows), and the cyst contains a chromatoid body with typically blunted ends (red arrow). The chromatoid body in this image is particularly well demonstrated.
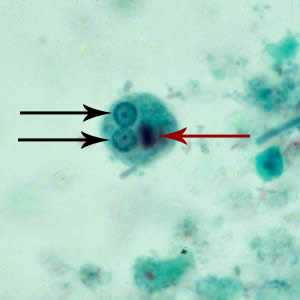
Figure D: Cyst of E. histolytica/E. dispar stained with trichrome. Two nuclei are visible in the focal plane (black arrows), and the cyst contains a chromatoid body with typically blunted ends (red arrow).
E. histolytica/E. dispar immature cysts stained with trichrome.
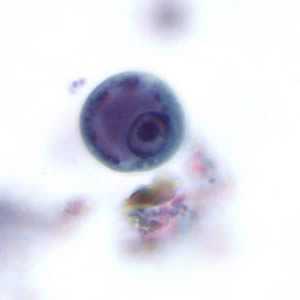
Figure A: Immature cyst of E. histolytica. The specimen was preserved in poly-vinyl alcohol (PVA) and stained with trichrome. PCR was performed on this specimen to differentiate between E. histolytica and E. dispar
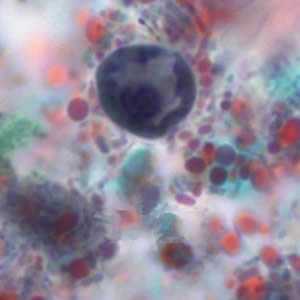
Figure B: Immature cyst of E. histolytica/E. dispar stained with trichrome. Image taken at 1000x oil magnification and contributed by the Kansas Department of Health and Environment.
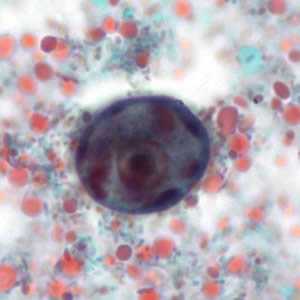
Figure C: Immature cyst of E. histolytica/E. dispar stained with trichrome. The cyst has large vacuoles and the chromatin around the nucleus is clumpy. These cysts range in size from 10.3 to 15.7 µm. Image taken at 1000x oil magnification and contributed by the Kansas Department of Health and Environment.
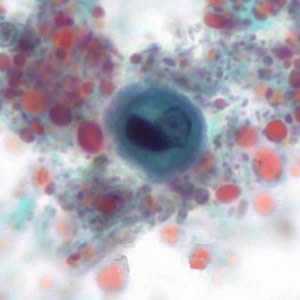
Figure D: Immature cyst of E. histolytica/E. dispar stained with trichrome. The cyst has large vacuoles and the chromatin around the nucleus is clumpy. These cysts range in size from 10.3 to 15.7 µm. Image taken at 1000x oil magnification and contributed by the Kansas Department of Health and Environment.
E. histolytica/E. dispar trophozoites in direct wet mounts.
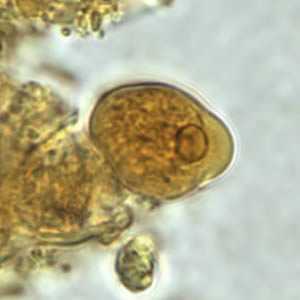
Figure A: Trophozoite of E. histolytica/E. dispar in a direct wet mount stained with iodine.
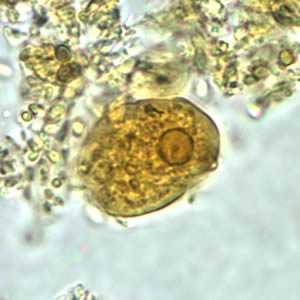
Figure B: Trophozoite of E. histolytica/E. dispar in a direct wet mount stained with iodine.
Entamoeba histolytica/Entamoeba dispar trophozoites stained with trichrome.
Entamoeba histolytica/Entamoeba dispar trophozoites have a single nucleus, which have a centrally placed karyosome and uniformly distributed peripheral chromatin. This typical appearance of the nucleus is not always observed as some trophozoites can have nuclei with an eccentric karyosome and unevenly distributed peripheral chromatin. The cytoplasm has a granular or "ground-glass" appearance. E. histolytica/E. dispar trophozoites usually measure 15 to 20 µm (range 10 to 60 µm), tending to be more elongated in diarrheal stool.
Erythrophagocytosis (ingestion of red blood cells by the parasite) is the only morphologic characteristic that can be used to differentiate E. histolytica from the nonpathogenic E. dispar. However, erthrophagocytosis is not typically observed on stained smears of E. histolytica.
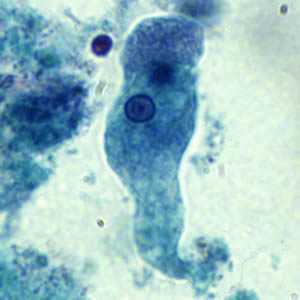
Figure A: Trophozoite of E. histolytica/E. dispar stained with trichrome.
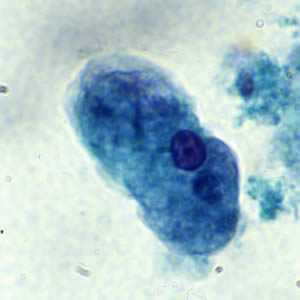
Figure B: Trophozoite of E. histolytica/E. dispar stained with trichrome.
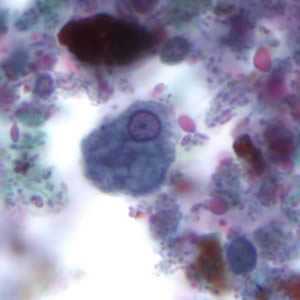
Figure C: Trophozoite of E. histolytica. The specimen was preserved in poly-vinyl alcohol (PVA) and stained with trichrome. PCR was performed on this specimen to differentiate between E. histolytica and E. dispar.
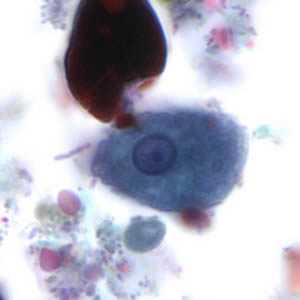
Figure D: Trophozoite of E. histolytica. The specimen was preserved in poly-vinyl alcohol (PVA) and stained with trichrome. The vacuolated cytoplasm seen in this image may be the result of less than optimal preservation. PCR was performed on this specimen to differentiate between E. histolytica and E. dispar.
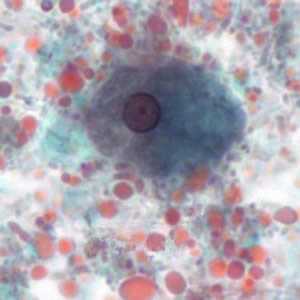
Figure E: Trophozoite of E. histolytica/E. dispar, measuring approximately 16.7 µm, stained with trichrome. The image was taken at 1000× magnification and contributed by the Kansas Department of Health and Environment.
Trophozoites of E. histolytica with ingested erythrocytes.
Entamoeba histolytica/Entamoeba dispar trophozoites have a single nucleus, which have a centrally placed karyosome and uniformly distributed peripheral chromatin. This typical appearance of the nucleus is not always observed as some trophozoites can have nuclei with an eccentric karyosome and unevenly distributed peripheral chromatin. The cytoplasm has a granular or "ground-glass" appearance. E. histolytica/E. dispar trophozoites usually measure 15 to 20 µm (range 10 to 60 µm), tending to be more elongated in diarrheal stool.
Erythrophagocytosis (ingestion of red blood cells by the parasite) is the only morphologic characteristic that can be used to differentiate E. histolytica from the nonpathogenic E. dispar. However, erthrophagocytosis is not typically observed on stained smears of E. histolytica.
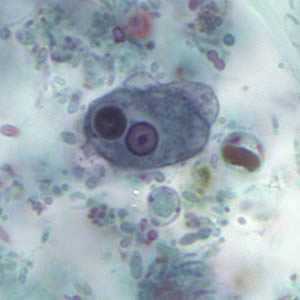
Figure A: Trophozoite of E. histolytica with ingested erythrocytes stained with trichrome. The ingested erythrocytes appear as dark inclusions. The parasite above shows nuclei that have the typical small, centrally located karyosome, and thin, uniform peripheral chromatin.
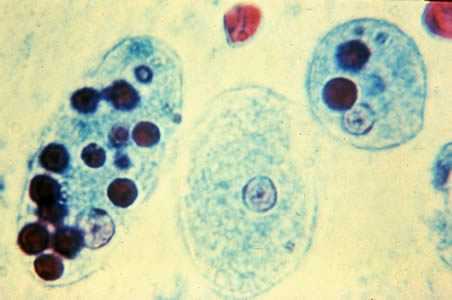
Figure B: Trophozoites of E. histolytica with ingested erythrocytes stained with trichrome. The ingested erythrocytes appear as dark inclusions. The parasites above show nuclei that have the typical small, centrally located karyosome, and thin, uniform peripheral chromatin.
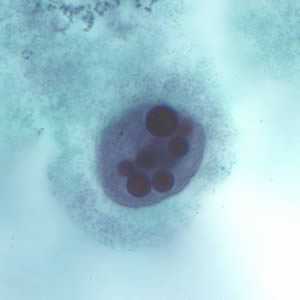
Figure C: Trophozoite of E. histolytica with ingested erythrocytes stained with trichrome.
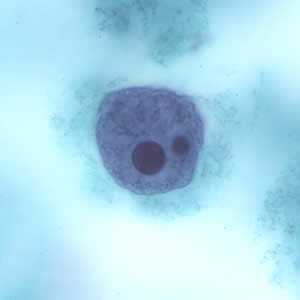
Figure D: Trophozoite of E. histolytica with ingested erythrocytes stained with trichrome.
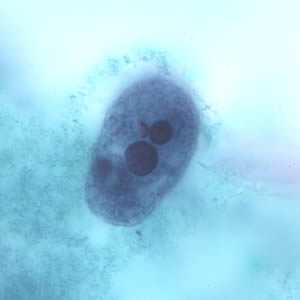
Figure E: Trophozoite of E. histolytica ingested erythrocytes stained with trichrome.
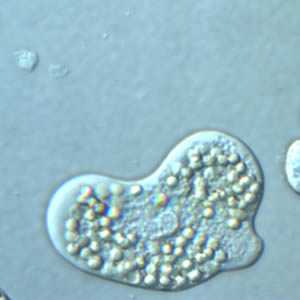
Figure F: Trophozoite of E. histolytica with ingested erythrocytes, under differential interference contrast (DIC) microscopy.
E. histolytica trophozoites in tissue stained with hematoxylin and eosin (H&E).
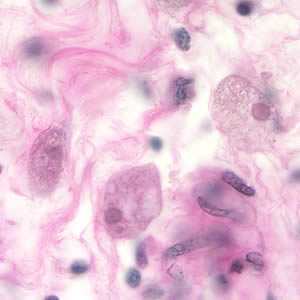
Figure A: Entamoeba histolytica trophozoites in colon tissue stained with H&E.
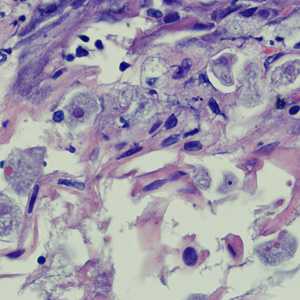
Figure B: Entamoeba histolytica trophozoites in a perianal biopsy, stained with H&E.
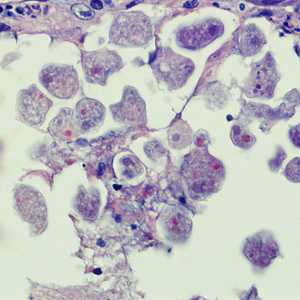
Figure C: Entamoeba histolytica trophozoites in a perianal biopsy, stained with H&E.
Laboratory Diagnosis
Entamoeba histolytica must be differentiated from other intestinal protozoa including: E. coli, E. hartmanni, E. gingivalis, Endolimax nana, and Iodamoeba buetschlii (the nonpathogenic amebae); Dientamoeba fragilis (which is a flagellate not an ameba); and the possibly pathogenic Entamoeba polecki. Differentiation is possible, but not always easy, based on morphologic characteristics of the cysts and trophozoites. The nonpathogenic Entamoeba dispar, however, is morphologically identical to E. histolytica, and differentiation must be based on isoenzymatic or immunologic analysis. Molecular methods are also useful in distinguishing between E. histolytica and E. dispar and can also be used to identify E. polecki. Microscopic identification of cysts and trophozoites in the stool is the common method for diagnosing E. histolytica. This can be accomplished using:
- Fresh stool: wet mounts and permanently stained preparations (e.g., trichrome).
- Concentrates from fresh stool: wet mounts, with or without iodine stain, and permanently stained preparations (e.g., trichrome). Concentration procedures, however, are not useful for demonstrating trophozoites.
In addition, E. histolytica trophozoites can also be identified in aspirates or biopsy samples obtained during colonoscopy or surgery.
Immunodiagnosis
Antibody Detection
Enzyme immunoassay (EIA) kits for Entomoeba histolytica antibody detection as well as EIA kits for antigen detection are commercially available in the United States. Antibody detection is most useful in patients with extraintestinal disease (i.e., amebic liver abscess) when organisms are not generally found on stool examination. Antigen detection may be useful as an adjunct to microscopic diagnosis in detecting parasites and can distinguish between pathogenic and nonpathogenic infections.
The indirect hemagglutination (IHA) test has been replaced by commercially available EIA test kits for routine serodiagnosis of amebiasis. Antigen consists of a crude soluble extract of axenically cultured organisms. The EIA test detects antibody specific for E. histolytica in approximately 95% of patients with extraintestinal amebiasis, 70% of patients with active intestinal infection, and 10% of asymptomatic persons who are passing cysts of E. histolytica. If antibodies are not detectable in patients with an acute presentation of suspected amebic liver abscess, a second specimen should be drawn 7-10 days later. If the second specimen does not show seroconversion, other agents should be considered. Detectable E. histolytica-specific antibodies may persist for years after successful treatment, so the presence of antibodies does not necessarily indicate acute or current infection. Specificity is 95% or higher: false-positive reactions rarely occur. Although the immunodiffusion test is as specific, it is slightly less sensitive than the IHA and EIA and requires a minimum of 24 hours to obtain a result, in contrast to 2 hours required for the IHA or EIA tests. However, the simplicity of the procedure makes it ideal for the laboratory that has only an occasional specimen to test. The IHA and EIA tests are more suitable for laboratories that have frequent requests for amebiasis serology. Although detection of IgM antibodies specific for E. histolytica has been reported, sensitivity is only about 64% in patients with current invasive disease. Several commercial EIA kits for antibody detection are available in the United States.
Antigen Detection
Antigen detection may be useful as an adjunct to microscopic diagnosis in detecting parasites and to distinguish between pathogenic and nonpathogenic infections. Recent studies indicate improved sensitivity and specificity of fecal antigen assays with the use of monoclonal antibodies which can distinguish between E. histolytica and E. dispar infections. At least one commercial kit is available which detects only pathogenic E. histolytica infection in stool; several kits are available which detect E. histolytica antigens in stool but do not exclude E. dispar infections.
References:
- Haque R, Ali IKM, Akther S, Petri WA Jr. Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. J Clin Microbiol 1998;36:449-452.
- Healy, GR. Serology. In: Ravdin JI, ed. Amebiasis: human infection by Entamoeba histolytica. New York: John Wiley & Sons; 1988. p. 650-719.
Molecular Diagnosis
Conventional PCR
In reference diagnosis laboratories, molecular analysis by PCR-based assays is the method of choice for discriminating between the pathogenic species (E. histolytica) and the nonpathogenic species (E. dispar).
Agarose gel (2%) analysis of a PCR diagnostic test for differentiation between E. histolytica and E. dispar.
- Lanes 1 - 4: Amplification with the Psp3/Psp51 PCR primer pair specific for E. histolytica. Diagnostic band size - 876 bp.
- Lanes 6 - 9: Amplification with the NPsp3/NPsp51 PCR primer pair specific for E. dispar. Diagnostic band size - 876 bp.
- Lanes 1 and 6: E. histolytica 200:NIH, zymodeme II (positive control for E. histolytica).
- Lanes 2 and 7: E. dispar 351:IMMiT, zymodeme I (positive control for E. dispar).
- Lanes 3 and 8: Specimen from a patient with a liver abscess (positive with E. histolytica primers and negative with E. dispar primers). E. histolytica 333:IMMiT, zymodeme XIV.
- Lanes 4 and 9: Specimen from an asymptomatic patient (positive with E. dispar primers and negative with E. histolytica primers). E. dispar 389:IMMiT, zymodeme I.
- Lane 5: Molecular base pair standard, 100-bp ladder (from 600 to 1,000 bp).
Real-Time PCR
A TaqMan real-time PCR approach has been validated at CDC and is used for differential laboratory diagnosis of amebiasis.2 The assay targets the 18S rRNA gene with species-specific TaqMan probes in a duplex format, making it possible to detect both species in the same reaction vessel.
More on: TaqMan real-time PCR
**** I made the link to TaqMan real time PCR more 508 and internet friendly ****
References:
- Clark CG, Diamond LS. Differentiation of pathogenic Entamoeba histolytica from other intestinal protozoa by riboprinting. Arch Med Res 1992;23:15-16.
- Qvarnstrom Y, James C, Xayavong M, Holloway B, Moura I, Visvesvara GS, et al. Comparison of real-time PCR rationales for differential laboratory diagnosis of amebiasis. J Clin Microbiol 2005;43:5491-5497.

Figure contributed by Assist. Prof. Przemyslaw Myjak, Ph.D., Institute of Maritime and Tropical Medicine, Gdynia, Poland.
Treatment Information
For asymptomatic infections, paromomycin or iodoquinol are the drugs of choice. For symptomatic intestinal disease or extraintestinal infections (e.g., hepatic abscess), the drugs of choice are metronidazole or tinidazole, immediately followed by treatment with paromomycin or iodoquinol.
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir
 Laboratory Diagnosis of Amebiasis -- Entamoeba histolytica and Entamoeba dispar
Laboratory Diagnosis of Amebiasis -- Entamoeba histolytica and Entamoeba dispar