Varicella Active Surveillance Project
On this Page
 From 1995 through 2010, the Varicella Active Surveillance Project (VASP) provided important data necessary for monitoring impact of the varicella vaccination program. VASP was established in 1994, when national varicella surveillance was not available, as a cooperative agreement funded by the Centers for Disease Control and Prevention (CDC) and implemented by the Philadelphia Department of Public Health and the Los Angeles County Department of Health Services from 1995 through 2010. The purpose of the project was (1) to obtain population-based incidence rates for varicella and herpes zoster and (2) to evaluate the impact of varicella vaccination practices and policies. In addition to active surveillance, numerous epidemiological studies were conducted throughout the years of the project and findings helped with our understanding of varicella epidemiology during the vaccination program. By 2010, impact from the varicella vaccination program demonstrated large declines in the number of varicella cases in the active surveillance sites such that they were no longer adequate for evaluating further impact of the program.
From 1995 through 2010, the Varicella Active Surveillance Project (VASP) provided important data necessary for monitoring impact of the varicella vaccination program. VASP was established in 1994, when national varicella surveillance was not available, as a cooperative agreement funded by the Centers for Disease Control and Prevention (CDC) and implemented by the Philadelphia Department of Public Health and the Los Angeles County Department of Health Services from 1995 through 2010. The purpose of the project was (1) to obtain population-based incidence rates for varicella and herpes zoster and (2) to evaluate the impact of varicella vaccination practices and policies. In addition to active surveillance, numerous epidemiological studies were conducted throughout the years of the project and findings helped with our understanding of varicella epidemiology during the vaccination program. By 2010, impact from the varicella vaccination program demonstrated large declines in the number of varicella cases in the active surveillance sites such that they were no longer adequate for evaluating further impact of the program.
History of VASP
The Varicella Active Surveillance Project (VASP) is a cooperative agreement funded by the Centers for Disease Control and Prevention (CDC) in September 1994 to
- develop a reporting system to accurately define the baseline incidence and epidemiological profile of varicella disease prior to licensure and wide use of varicella vaccine,
- identify changes in the epidemiology of varicella as a result of vaccine usage,
- ascertain the immunization status of cases, and
- evaluate the demographic and clinical profiles of vaccinated and unvaccinated cases of varicella.
Originally 3 areas were under surveillance: Travis County, TX, Antelope Valley, CA, and West Philadelphia, PA. Currently, only Antelope Valley (implemented by the Los Angeles County Health Department) and West Philadelphia (implemented by the Philadelphia Department of Public Health) project areas were under surveillance for the entire project period, 1995 through 2010.
Purpose
- Implement, conduct, maintain, and evaluate active population-based surveillance systems with capacity to monitor varicella and herpes zoster disease.
- Perform case investigations for varicella and herpes zoster for all ages and collect, analyze and disseminate information using these data.
- Collect and report information on vaccine doses administered by age group.
- Develop, implement and evaluate varicella prevention and control strategies including outbreak control.
- Provide laboratory specimens for laboratory evaluation needed for varicella and herpes zoster surveillance or as part of epidemiological studies, e.g., virus strain identification, confirmation of breakthrough disease, and molecular epidemiological studies.
- Conduct applied epidemiological studies for varicella and herpes zoster diseases to contribute to the immunization program policy and guidelines.
Methodology
Reporting Sites
- There were 3 original surveillance areas (Travis County, TX, Antelope Valley, CA, and West Philadelphia, PA), but only the Antelope Valley, CA and West Philadelphia, PA participated for the entire surveillance project period (1995-2010).
- Approximately 300 participating reporting sites were included in each surveillance area, consisting of hospitals, public and private schools, primary care practitioners, public health clinics, licensed child-care facilities, prisons, homeless shelters, and universities.
Case Identification
- Case identification was facilitated through a standardized surveillance system.
- Each surveillance site reported twice a month to VASP the presence or absence of cases of varicella and herpes zoster within their facility.
- Billing records were obtained from local healthcare systems and public health clinics to identify cases which may not have been reported during routine surveillance.
Case Interview
- After notification, each patient, or his/her parent/guardian, was interviewed via telephone or house visit to confirm diagnosis and to obtain detailed clinical and demographic information.
- The interviewer assessed whether there were additional cases or susceptible contacts within the household.
- A case investigation was completed for each newly identified case of varicella.
- To improve case ascertainment, all household contacts without a positive history of varicella disease were re-contacted in 3 weeks (one incubation period) after the onset of the most recent case to investigate potential household spread of varicella infection. If there was more than one susceptible contact living within a household, the contacts were followed for 6 weeks or 2 incubation periods.
Herpes Zoster Surveillance
- VASP conducted surveillance for cases of herpes zoster among individuals <20 years during 2000-2010 and among individuals ≥50 years during 2006-2010.
- VASP collected data on herpes zoster cases among children and adolescents who were less than <20 years of age to 1) measure the burden of herpes zoster after implementation of the varicella vaccination program, 2) compare the clinical presentation of herpes zoster in children with wild-type varicella disease as compared with herpes zoster among children who received the varicella vaccine, and 3) evaluate the relative risk of developing herpes zoster among children vaccinated with the varicella vaccine as compared to unvaccinated children.
Laboratory Testing
- All specimens were sent to CDC's National Varicella Zoster Virus (VZV) Laboratory for testing. Common tests performed included: PCR, VZV IgG gpELISA, and VZV IgM ELISA.
Results
Increase in varicella vaccination coverage
- In Los Angeles County, one-dose vaccination coverage among children between the ages of 19 and 35 months increased from 37.9% in 1997 to 82.1% in 2000, to 90.5% in 2006, and 95.1% in 2010.
- In Philadelphia, vaccination coverage increased from 41.2% in 1997, to 83.8% in 2000, to 92.7% in 2006, and 94.6% in 2010.
Decline in varicella cases
- VASP was the first source of data to document the impact of the varicella vaccination program on varicella cases and incidence.
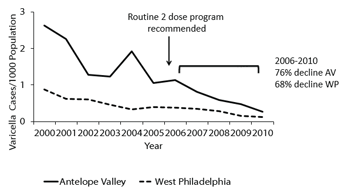
- Between 1995 and 2000, the total number of cases in the three participating surveillance areas (Antelope Valley, California, West Philadelphia, Pennsylvania and Travis County, Texas) declined 71% to 84%, with the most considerable reduction in preschool children (1-4 year olds).
- By 2010, varicella incidence declined 98% in the two remaining sites (Antelope Valley, California and West Philadelphia, Pennsylvania) compared with 1995.
- Among infants, varicella disease decreased 90% during 1995 to 2008, and among adults, varicella disease decreased 74% during 1995-2005.
- During the 2-dose vaccination period, incidence declined between 67% and 76%. Overall incidence rates during 2005-2010 calculated using confirmed/probable cases were similar in these active surveillance sites as compared to national data from passive surveillance areas.
View larger image
Published in Pediatrics: Bialek SR, Perella D, Zhang J, Mascola L, Viner K, Jackson C, et al. Impact of a Routine Two-Dose Varicella Vaccination Program on Varicella Epidemiology. Pediatrics. Nov 2013, 132 (5) e1134-e1140; DOI: 10.1542/peds.2013-0863.
Decline in varicella outbreaks
- From 1995-1998 to 2002-2005, varicella outbreaks significantly decreased in number, from 236 to 46 in Antelope Valley. Varicella outbreaks also decreased in size (as measured by number of varicella cases), from a median of 15 cases to 9 cases per outbreak, and in duration, from 44.5 days to 30 days.
- During 2007-2010, after the recommendation for routine 2-doses of varicella vaccine, 12 varicella outbreaks were reported in Antelope Valley, representing a 95% decline in outbreaks as compared to 1995-1998.
Decline in varicella hospitalizations
- From 1995 to 1998, hospitalization rates ranged from 2.2 to 3.3 per 100,000 population and decreased to 0.5 per 100,000 in 2004 and 0.8 per 100,000 in 2005.
- During 2006-2010, the hospitalization rate declined to 0.2 per 100,000 in Antelope Valley and 0.5 per 100,000 in Philadelphia. This represents a 92% decline in varicella hospitalizations in Antelope Valley and an 87% decline in West Philadelphia in 2006-2010 as compared to 1995-1998.
Herpes Zoster among persons <20 years of age
- The incidence of herpes zoster among children <10 years of age declined by 69% when comparing 2000-2006 with 2007-2010.
- The incidence of herpes zoster among 10- to 19-year-olds fluctuated substantially during 2000-2010 with an overall 13% increase during 2007-2010 as compared with 2000-2006. A 63% increase was reported between 2000 and 2006. After 2006, lower rates were observed in 3 of the 4 additional years analyzed.
- Among children aged<10 years during 2000-2006, those with a history of varicella vaccination had a 4 to 12 times lower risk for developing herpes zoster compared with children with history of varicella disease.
References
- Bialek SR, Perella D, Zhang J, Mascola L, Viner K, Jackson C, Lopez AS, Watson B, Civen R. Impact of a routine two-dose varicella vaccination program on varicella epidemiology. Pediatrics. 2013 Nov;132(5):e1134-40.
- CDC. National and state vaccination coverage among children aged 19-35 months--United States, 2010. MMWR. 2011 Sep 2;60(34):1157-63.
- CDC. National Immunization Survey (NIS)-Children (19-35 months).
- Chaves SS, Lopez AS, Watson TL, Civen R, Watson B, Mascola L, Seward JF. Varicella in infants after implementation of the US varicella vaccination program. Pediatrics. 2011 Dec;128(6):1071-7.
- Civen R, Chaves SS, Jumaan A, Wu H, Mascola L, Gargiullo P, Seward JF. The incidence and clinical characteristics of herpes zoster among children and adolescents after implementation of varicella vaccination. Pediatr Infect Dis J. 2009 Nov;28(11):954-9.
- Civen R, Lopez AS, Zhang J, Garcia-Herrera J, Schmid DS, Chaves SS, Mascola L. Varicella outbreak epidemiology in an active surveillance site, 1995-2005. J Infect Dis. 2008 Mar 1;197 Suppl 2:S114-9.
- Guris D, Jumaan AO, Mascola L, Watson BM, Zhang JX, Chaves SS, et al. Changing varicella epidemiology in active surveillance sites—United States, 1995-2005. J Infect Dis. 2008;197(Suppl 2):S71-5.
- Marin M, Civen R, Zhang JQ, Abraham A, Harpaz R, Mascola L, Bialek SR. Update on incidence of herpes zoster among children and adolescents following implementation of varicella vaccination, Antelope Valley, CA, 2000- 2010. IDweek, San Diego, CA, October 7-11, 2015
- Marin M, Watson TL, Chaves SS, Civen R, Watson BM, Zhang JX, Perella D, Mascola L, Seward JF. Varicella among adults: data from an active surveillance project, 1995-2005. J Infect Dis. 2008 Mar 1;197 Suppl 2:S94-S100.
- Reynolds MA, Watson BM, Plott-Adams KK, Jumaan AO, Galil K, Maupin TJ, et al. Epidemiology of varicella hospitalizations in the United States, 1995-2005. J Infect Dis. 2008;197(Suppl 2):S120-6.
- Seward JF, Watson BM, Peterson CL, Mascola L, Pelosi JW, Zhang JX, Maupin TJ, Goldman GS, Tabony LJ, Brodovicz KG, Jumaan AO, Wharton M. Varicella disease after introduction of varicella vaccine in the United States, 1995-2000. JAMA. 2002 6;287(5):606-11.
- Viner K, Perella D, Lopez A, Bialek S, Nguyen M, Spells N, Watson B. Comparing active and passive varicella surveillance in Philadelphia, 2005-2010: recommendations for the transition to nationwide passive varicella disease surveillance. Public Health Rep. 2014 Jan-Feb;129(1):47-54.
- Page last reviewed: July 1, 2016
- Page last updated: July 1, 2016
- Content source:


 ShareCompartir
ShareCompartir