Laboratory Guidance and Diagnostic Testing
Dengue can be diagnosed by isolation of the virus, by serological tests, or by molecular methods. Diagnosis of acute (on-going) or recent dengue infection can be established by testing serum samples during the first 5 days of symptoms and/or early convalescent phase (more than 5 days of symptoms). Acute infection with dengue virus is confirmed when the virus is isolated from serum or autopsy tissue specimens, or the specific dengue virus genome is identified by reverse transcription-polymerase chain reaction (RT–PCR) from serum or plasma, cerebrospinal fluid, or autopsy tissue specimens during an acute febrile illness. Methods such as one-step, real time RT–PCR or nested RT–PCR are now widely used to detect dengue viral genes in acute-phase serum samples. This detection coincides with the viremia and the febrile phase of illness onset. Acute infections can also be laboratory confirmed by identification of dengue viral antigen or RNA in autopsy tissue specimens by immunofluorescence or immunohistochemical analysis, or by seroconversion from negative to positive IgM antibody to dengue or demonstration of a fourfold or greater increase in IgG antibody titers in paired (acute and convalescent) serum specimens.
Patients who have IgM antibodies to dengue detected in their serum specimen via an IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA) and had either 1.) A negative RT–PCR result in the acute phase specimen or 2.) Did not submit an acute phase specimen, are classified as having a recent probable dengue infection. This is due to the fact that IgM antibodies for dengue may remain elevated for 2 to 3 months after the illness. The elevated IgM observed in a sample could be the result of an infection that occurred 2 to 3 months ago. In addition, there is cross reactivity with other flaviviruses including West Nile virus (WNV), St. Louis encephalitis virus (SLE), Japanese encephalitis virus (JEV) and yellow fever virus (YFV). The provider should review the patient’s past medical history, recent travel history, and vaccination record (especially yellow fever vaccination) to determine the likelihood that the current acute febrile illness is due to an infection with dengue virus.
Often times both an acute and convalescent phase specimens are needed to make a diagnosis of dengue infection. This is especially true for those who submit a day 5 acute specimen because the virus and IgM antibodies may be at undetectable levels. So if a patient with suspected dengue infection submits a late acute phase specimen that is negative (e.g., by RT–PCR and MAC-ELISA), and they do not submit a convalescent specimen, they are classified as a laboratory-indeterminate case.
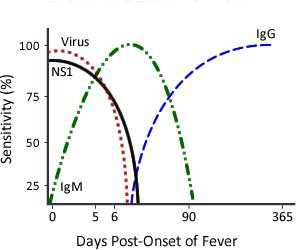
- Immunological Response to Dengue InfectionThe acquired immune response following a dengue infection consists of the production of IgM and IgG antibodies primarily directed against the virus envelope proteins. The immune response varies depending on whether the individual has a primary (first dengue or other flavivirus infection) versus a secondary (had dengue or other flavivirus infection in past) dengue infection. In general, diagnosis of dengue is dependent on the phase of the infection. The general timeline of a primary infection from virus isolation or identification, to IgM detection followed by IgG detection is as follows:
Legend
 DENV-reactive IgG
DENV-reactive IgG
DENV-reactive IgM
Dengue viral protein, NS1
Primary DENV Infection
Secondary DENV Infection
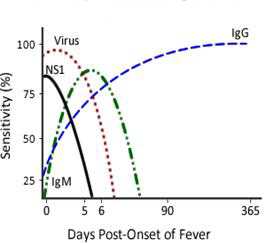
A primary dengue infection is characterized by a slow and low titer antibody response. IgM antibody is the first immunoglobulin isotype to appear. Anti-dengue IgG is detectable at low titer at the end of the first week of illness, and slowly increases. In contrast, during a secondary infection, antibody titers rise extremely rapidly and antibody reacts broadly with many flaviviruses. High levels of IgG are detectable even in the acute phase and they rise dramatically over the proceeding two weeks. The kinetics of the IgM response is more variable. IgM levels are significantly lower in secondary dengue infections and thus some anti-dengue IgM false-negative reactions are observed during secondary infections. According to the Pan American Health Organization (PAHO) guidelines 80% of all dengue cases have detectable IgM antibody by day five of illness, and 93-99% of cases have detectable IgM by day six to ten of illness, which may then remain detectable for over 90 days.
MAC-ELISA has become an important tool for routine dengue diagnosis, MAC-ELISA has a sensitivity and specificity of approximately 90% and 98%, respectively but only when used five or more days after onset of fever (i.e., in convalescent phase). Different formats such as capture ELISA, capture ultramicroELISA, dot-ELISA, AuBioDOT IgM capture and dipsticks have been developed. Serums, blood on filter paper, and saliva (but not urine) are useful for IgM detection if samples are taken in convalescent phase of illness (Vasquez et al., 2006). A variety of different commercial kits is available with variable sensitivity and specificity. Dengue diagnosis becomes even more challenging because dengue IgM antibodies also cross-react to some extent with other flaviviruses such asJEV, SLE, WNV and YFV.
-
Testing Algorithms for Dengue:
- PCR
DENV can be detected in the blood (serum) from patients for approximately the first 5 days of symptoms. Currently, several PCR tests are employed to detect the viral genome in serum. In addition, virus can be isolated and sequenced for additional characterization. Real time RT–PCR assays have been developed and automated; but none of these tests are yet commercially available. Because antibodies are detected later, RT–PCR has become a primary tool to detect virus early in the course of illness. Current tests are between 80-90% sensitive, and more that 95% specific. A positive PCR result is a definite proof of current infection and it usually confirms the infecting serotype as well. However, a negative result is interpreted as “indeterminate”. Patients receiving negative results before 5 days of illness are usually asked to submit a second serum sample for serological confirmation after the 5th day of illness (bellow). - MAC ELISA
IgM antibody capture ELISA (MAC-ELISA) format is most commonly employed in diagnostic laboratories and commercial available diagnostic kits. The assay is based on capturing human IgM antibodies on a microtiter plate using anti-human-IgM antibody followed by the addition of dengue virus specific antigen (DENV1-4). The antigens used for this assay are derived from the envelope protein of the virus. One of the limitation of this testing is the cross reactivity between other circulating flaviviruses. This limitation must be considered when working in regions where multiple flaviviruses co-circulate. IgM detection is not useful for dengue serotype determination due to cross-reactivity of the antibody. - IgG ELISA
The IgG ELISA used for the detection of a past dengue infection utilizes the same viral antigens as the MAC ELISA. This assay correlates with the hemagglutination assay (HI) previously used. In general IgG ELISA lacks specificity within the flavivirus serocomplex groups. Primary versus secondary dengue infection can be determined using a simple algorithm. Samples with a negative IgG in the acute phase and a positive IgG in the convalescent phase of the infection are primary dengue infections. Samples with a positive IgG in the acute phase and a 4 fold rise in IgG titer in the convalescent phase (with at least a 7 day interval between the two samples) is a secondary dengue infection. - NS1 ELISA
The non-structural protein 1 (NS1) of the dengue viral genome has been shown to be useful as a tool for the diagnosis of acute dengue infections. Dengue NS1 antigen has been detected in the serum of DENV infected patients as early as 1 day post onset of symptoms (DPO), and up to 18 DPO. The NS1 ELISA based antigen assay is commercially available for DENV and many investigators have evaluated this assay for sensitivity and specificity. The NS1 assay may also be useful for differential diagnostics between flaviviruses because of the specificity of the assay. - PRNT
Plaque Reduction and Neutralization Test (PRNT) and the microneutralization PRNT can be used when a serological specific diagnostic is required, as this assay is the most specific serological tool for the determination of dengue antibodies The PRNT test is used to determine the infecting serotype in convalescent sera. This assay measures the titer of the neutralizing antibodies in the serum of the infected individual and determines the level of protective antibodies this individual has towards the infecting virus. The assay is a biological assay based on the principle of interaction of virus and antibody resulting in inactivation of virus such that it is no longer able to infect and replicate in cell culture. Some of the variability of this assay is differences in interpretation of the results because of the cell lines and virus seeds used as well as the dilution of the sera.
- PCR
The microneutralization assay is based on the same principle however instead of counting the number of plaques per well the assay uses a colorimetric measurement of the virus induced cell lysis to determine the end-point dilution. This assay was developed to utilize less reagents and for high throughput purposes for larger number of samples for testing.
Requesting dengue laboratory testing and case reporting
- Instructions for collecting, preparing and mailing specimens for dengue testing at the CDC Dengue Laboratory English [PDF – 7 pages] and (en Español) [DOC – 5 pages]
- WNV case report form for PR (en español) [PDF – 1 page]
- Hospitals, laboratories and health care centers in Puerto Rico that will draw serum samples in Puerto Rico. [DOC – 1 page]
- Page last reviewed: July 8, 2010
- Page last updated: August 3, 2017
- Content source:


 ShareCompartir
ShareCompartir
