Chapter 8: Health Effects Evaluation: In-Depth Analysis
- (Section 8.1) When to Conduct an In-depth Analysis
- (Section 8.2) Tools and Resources Needed to Support an In-depth Analysis
- (Section 8.3) Evaluating Study(ies) on Which Exceeded Health Guidelines are Based
- (Section 8.4) Reviewing Other Dose-response Data
- (Section 8.5) Evaluating Substance-specific Factors that Can Increase or Decrease the Potential for Harm
- (Section 8.6) Evaluating Site-specific Health Effects Data
- (Section 8.7) Presenting Findings in the Public Health Assessment Document
As part of the exposure evaluation (described in Chapters 5 and 6), you have identified who might come in contact with environmental contaminants, how those persons might be exposed, and the extent to which might be exposed (over space and time). As an initial step in the health effects evaluation (described in Chapter 7), you have compared, measured, or modeled exposure point concentrations to ATSDR's media-specific comparison values. In some cases, you have estimated site-specific exposure doses and compared them to health guidelines. By now, you have clearly ruled out those pathways and substances that pose no health hazards, and you have retained those requiring more careful examination.
This chapter provides guidance on how to perform the more in-depth analysis needed at sites where, during the exposure evaluation and screening analysis, health hazards have not been ruled out. To this point in the public health assessment process—with the exception of knowing the numeric value of the health-based comparison value—no information about the substance(s) of interest has been required. As depicted in Figure 8-1, the process described in this chapter involves looking more closely at substance-specific information in the context of site exposures. The goal of this analysis is to provide perspective on what it means when a health-based screening value has been exceeded, and in some cases, how to address specific community health concerns regarding that situation. The analysis will help answer two important questions health assessors face:
- Are public health actions needed to prevent exposures?
- Are site-related exposures expected to cause harm?
This chapter will guide you in evaluating and integrating exposure data (i.e., site-specific exposure conditions that have been studied throughout the public health assessment process) and substance-specific health effects data (e.g., toxicologic, epidemiologic, and health outcome data). The output of the analysis is a qualitative description of whether site exposure conditions are of sufficient nature, frequency, and magnitude to affect public health adversely. The outcome will also assist in determining an appropriate public health response.
Because of uncertainties regarding exposure conditions and the adverse effects associated with environmental levels of exposure, definitive answers on whether health effects actually will or will not occur are not always possible. However, providing a framework that puts site-specific exposures and the potential for harm in perspective is possible and is one of the primary goals of the public health assessment process. The narrative describing your findings should therefore lay out this framework.
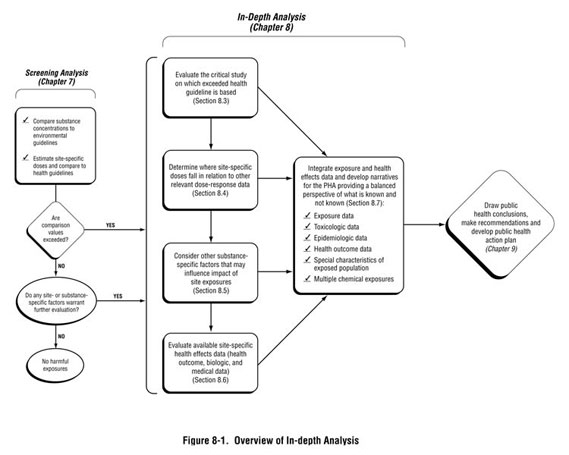
After discussing the criteria that will trigger an in-depth analysis (Section 8.1) and the tools and resources available to support your analysis (Section 8.2), this chapter will guide the health assessor through the following steps:
- Evaluating the experimental or human study(ies) on which the exceeded health guideline value was based. (Section 8.3)
- Determining where site-specific dose estimates fall in relation to other dose-response data. (Section 8.4)
- Reviewing other substance-specific factors that could increase or decrease the potential for harmful effects, such as our understanding of the overall behavior of the substance within the human body and the mechanism by which it exerts its toxic effect, knowledge of substance-specific effects among susceptible populations, and multiple chemical exposures. (Section 8.5)
- Determining whether relevant site-specific health effects data should be evaluated in the public health assessment, such as mortality and morbidity data (also called health outcome data), or biologic monitoring data (Section 8.6).
- Integrating relevant information and presenting it in the PHA document. (Section 8.7)
Not all public health assessments will require you to consider all the elements of the in-depth analysis described in this chapter. The level of analysis will differ across sites and will depend on the scope and complexity of site-related issues, such as the magnitude of exposures, the substance(s) under evaluation, and specific community health concerns.
As you review and integrate exposure and health effects data, professional judgment is needed in weighing what is known and unknown, including uncertainties and data limitations. You may need assistance from other members of your site team or other technical specialists, including those with expertise in toxicology, epidemiology, medicine, and health physics. This chapter will guide you on how to work with these specialists to define the appropriate level of analysis for your site and in evaluating the strength and relevance of available information. As the health assessor, you will be responsible for integrating and communicating the findings of this analysis in the public health assessment document.
8.1 When to Conduct an In-depth Analysis
During the screening analysis (Chapter 7), after careful consideration of site-specific exposure conditions, you eliminated those substances and pathways not expected to result in adverse health effects. You then determined whether exposure to measured or modeled levels of contaminants required further evaluation. In many cases, you will not need to go any further. However, you should proceed with a more detailed analysis, as outlined in this chapter, if any of the following occur (1):
- Site-specific exposure dose estimates exceed health-based guideline values (e.g., MRLs are exceeded or theoretical cancer risk levels exceed 10-6).
- No relevant and reliable screening value could be found or generated for a substance. As noted in Chapter 7, exceptions can include essential nutrients and other constituents naturally found in environmental media (e.g., calcium, iron, magnesium).
- The community has expressed concern about a particular substance or exposure. Even in cases where comparison values have not been exceeded, a more in-depth review of the health effects data might be needed to adequately address the community health concern.
8.2 Tools and Resources Needed to Support an In-depth Analysis
In general, an in-depth analysis will require the examination and interpretation of reliable substance-specific health effects data (toxicologic, epidemiologic, medical, and health outcome data). Much of the data will relate to dose-response relationships for the substance and pathways of interest. You also will determine whether health outcome data should or can be obtained (i.e., information from pre-existing databases such as local or state disease registries). In some cases, community or site-specific survey data might be available for evaluation as part of the public health assessment.
ATSDR's toxicological profiles serve as an important resource for health effects data. In most cases, these profiles will provide the information needed to support your analysis and draw public health conclusions. Each peer-reviewed profile identifies and reviews the key literature that describes the toxicologic properties and adverse effects associated with a substance, including information on populations that might be unusually susceptible to a particular substance. These profiles also contain other substance-specific data, such as information on bioavailability and interaction with other chemicals. Limitations and uncertainties of individual studies and the overall database are highlighted. The box below summarizes the content of ATSDR's Toxicological Profiles.
Other compilations of toxicologic data include resources such as the U.S. Environmental Protection Agency's (EPA) Integrated Risk Information System (IRIS) database, International Agency for Research on Cancer (IARC) Monographs, and National Toxicology Program (NTP), as well as some non-governmental resources. For more in-depth evaluations or in the absence of secondary sources such as those mentioned above, standard toxicology textbooks and peer-reviewed scientific journals of environmental toxicology or environmental health can be consulted. A listing of and links to such resources are provided at the end of this chapter.
When identifying the most relevant and up-to-date sources of data to support your analysis, you might need to consult with the appropriate experts on your team. Conducting a critical review of toxicologic or epidemiologic data requires specialized training and a thorough understanding of underlying scientific principles. Similarly, a health physicist will need to assist in identifying appropriate resources for evaluating radiological hazards. The ATSDR Division of Toxicology chemical manager is another resource in determining the status of any ongoing substance-specific research. If available secondary resources (such as toxicological profiles) have not been recently updated, it is important to identify the current state of the knowledge for a particular substance. (While ATSDR is continually reviewing substance-specific toxicologic data, some of the profiles could be a few years old.) New information regarding observed effect levels or low-dose behavior might be important in interpreting site-specific doses (see sections that follow).
|
ATSDR's Toxicological Profiles ATSDR's Toxicological Profiles contain information for more than 200 chemicals (http://www.atsdr.cdc.gov/toxprofiles/index.asp) commonly found at hazardous waste sites. This includes "interaction profiles" for chemical mixtures that may be found together in environmental media at hazardous waste sites (e.g., arsenic, hydrazines, jet fuels, strontium, and trichloroethylene). In general, the profiles present:
Each profile presents a public health statement that answers basic health questions in plain language. In addition to including information on the chemical's use, physical/chemical properties, and pertinent regulations and advisories, each profile presents a detailed summary of the toxicology of the chemical through a review of the peer-reviewed literature, including an analysis of the adequacy of the database and the identification of data gaps. Note that Appendix B of each Toxicological Profile includes a User's Guide. Except in rare cases (e.g., PCBs), health effects are discussed by route of exposure (inhalation, oral, and dermal), by type of effect (death, systemic, immunologic and lymphoreticular, neurological, reproductive, developmental, genotoxic, and cancer), and by exposure duration (acute, subchronic, and chronic). Toxicokinetics (absorption, distribution, metabolism, elimination/excretion, and PBPK/PBPD models, when available) also are described. When information is available, the profile also discusses chemical mechanism of action and interactive effects with other chemicals. The profile includes a description of potentially sensitive or unusually susceptible populations, including children. Potential for human exposure (including discussions on environmental releases, typical environmental levels, and environmental fate), biomarkers of exposure and effect, and methods for measuring the chemical are detailed when possible. Each profile also presents the basis for any MRLs derived for that particular substance (including the study[ies] used, critical endpoint[s], and uncertainty factors applied). Health assessors are encouraged to consult with the chemical manager within ATSDR's Division of Toxicology to determine the status of substance-specific profiles and any ongoing research, especially for chemicals with profiles that have not been recently updated. |
8.3 Evaluating Studies on Which Exceeded Health Guidelines are Based
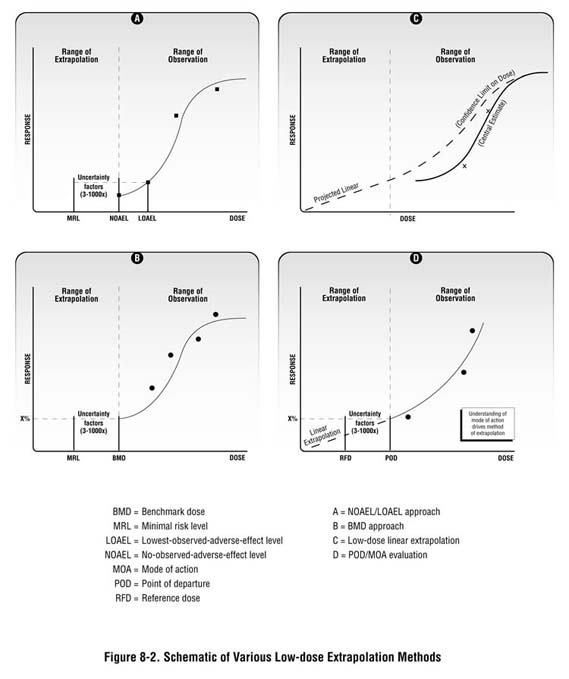
As described in Chapter 7 and in Appendix D, the health guidelines used in your screening analysis are generally extrapolated doses from observed effect levels in animal studies. Health guidelines are usually based on a "critical" or "key" study—generally, the study reporting the most sensitive endpoint at the lowest dose level. Depending on the available data and the type of toxic response, observed effect levels are then adjusted by a series of uncertainty factors or through the use of statistical models to ensure that they are amply health-protective (see Figure 8-2). Setting screening values at levels well below those known to cause harm is consistent with the fundamental concept of public health: prevention.
When a health guideline is exceeded, a first step in understanding the public health significance of exceeding that guideline is to review and understand the basis for that guideline. Understanding the applicability and strength of the study data will be a primary tool in evaluating whether site exposures are expected to cause harm. The goal of the analysis is to determine where site-specific doses lie in relation to the observed effects levels reported in the studies of interest and whether differences between study data and the exposure scenario being evaluated make health effects more or less likely.
When developing health guidelines such as MRLs, ATSDR toxicologists and others extensively study the toxicologic literature and weigh the scientific data (including the factors highlighted below). Reviewing the basis for an MRL and other health guidelines as part of this analysis in no way diminishes the importance of the health guideline; rather, it serves as a means of gaining perspective on how strongly the supporting toxicologic data suggest that harmful exposures have occurred or might occur under your site-specific exposure conditions.
| Simply being exposed to a hazardous substance does not make it a hazard. The magnitude, frequency, timing, and duration of exposure and the toxicity characteristics of individual substances affect the degree of hazard, if any. |
Two key steps in this analysis involve (1) comparing site exposure doses with observed effect levels reported in the critical study (Section 8.3.1) and (2) carefully considering study parameters in the context of site exposures (see Section 8.3.2). You will generally find information on the critical study in ATSDR's toxicological profiles, in the section entitled Health Effects: Relevance to Public Health and in the appendix presenting ATSDR Minimal Risk Levels and Worksheets, or in EPA's IRIS database.
8.3.1 Compare Site-Specific Doses to Observed Effect Levels
Non-cancer effects
This step in the process involves comparing your substance-specific exposure dose to effect levels that are reported in the critical study used to derive the screening value that has been exceeded. (2) The health assessor should review the section of the toxicological profile in which the MRLs are derived. The assessor should note whether the MRL is based on a human or animal study and whether the MRL is derived from a NOAEL or LOAEL. The assessor should then compare the site-specific exposure dose to the NOAEL or LOAEL. (3)
If the site-specific exposures are well below a NOAEL that is based on a human study, the likelihood for adverse health effects in the exposed population would be low. If, however, the NOAEL is based on an animal study, exposure doses near the NOAEL could be of concern because of uncertainty in the relative sensitivity of animals as compared to humans. (In the absence of contrary information, it is prudent to assume that humans are more sensitive to the chemical than are animals.)
In some instances, an MRL is derived from a LOAEL, rather than from a NOAEL. The likelihood of adverse health effects increases as site-specific exposures approach a LOAEL derived from either a human or animal study. Because, by definition, LOAEL doses cause adverse health effects, exposures that approach or exceed a LOAEL are of concern and should be identified as a public health hazard.
The health assessor should also consider the relevance of the MRL study to the site-specific exposure conditions and the exposed population. If the MRL was based on a NOAEL in adults, and the population at the site includes a sensitive population such as children, the NOAEL might not apply to all segments of the population. The assessor should also consider the exposure scenario of the MRL study. In experimental studies, administration of a high bolus dose of a chemical to an animal could have a different effect than low-dose chronic or intermittent exposures in humans. Also, the assessor should consider the confidence in the MRL study; if similar findings have been reported in other studies, confidence in the study is enhanced. Section 8.3.2 of this chapter discusses other factors to consider when evaluating the relevance of the MRL study.
As you review and integrate exposure and health effects data, professional judgment is needed in weighing what is known and unknown, including uncertainties and data limitations. You may need assistance from other members of your site team or other technical specialists, including those with expertise in toxicology, epidemiology, medicine, and health physics. The assessor is also encouraged to consult with other health assessors to gain insight into how similar situations have been addressed previously.
|
A Harmonized Approach for Assessing All Toxic Endpoints Historically, different approaches have been used in conducting quantitative risk assessment for non-cancer and cancer endpoints. For non-cancer risk assessment, the more traditional toxicology principle of dose "thresholds" has been applied when evaluating potential risks, where the potential for adverse health effects is evaluated based on relevant observed effect levels known as the "no-observed-adverse-effect level" (NOAEL) or the "lowest-observed-adverse-effect level" (LOAEL). For cancer risk assessment, on the other hand, the approach used by risk assessors has been to assume that no threshold exists. This stems back to early assumptions that the process of chemical carcinogenesis is similar to that of radiation carcinogenesis and that any exposure is assumed to carry with it a risk of cancer (Bogdanffy et al. 2001). Under this assumption, risk assessors extrapolate down to low doses using statistical models based on an assumed dose-response relationship (generally considered linear to zero). Such modeling can predict risks associated with doses thousands of times lower than those at which tumors are actually observed (referred to in ATSDR's toxicological profiles as "cancer effect levels"). Advancing scientific knowledge regarding the mechanism by which substances act at low doses suggests that the traditional use of threshold and non-threshold models for non-cancer and cancer risk assessment, respectively, needs to be re-examined (EPA 2003a). For example, scientists are learning that some carcinogens are not genotoxic (that is, cancer is not initiated by interaction with DNA). In such cases, threshold dose levels can be identified and used for comparison purposes in interpreting exposure doses, similar to our comparison to "NOAELs." Further, research on modes of chemical toxicity may establish links between non-cancer responses to toxic agents and subsequent overt manifestations of toxicity such as cancer (Bogdannffy et al. 2001). The guidance provided in this chapter is built on this broader understanding of toxic action. It guides the health assessor through a series of considerations related to toxic potential that will help determine whether the potential for harm is more or less likely given what is known and not known about the characteristics of a particular substance under site-specific exposure conditions. |
Cancer effects
In some cases, quantitative risk assessment might have been used in your screening analysis or by regulatory agencies evaluating your site. Regulators could call for cleanup of a site when theoretical cancer risks fall within the 10-6 to 10-4 range, but understanding the variables and assumptions involved in the derivation of these estimates and explaining in qualitative terms what exposure doses mean based on a review of the scientific literature is the purpose of the in-depth analysis.
As with all toxic endpoints, you need to look at site-specific doses in relation to observed effect levels and then provide context. Consider each of the following factors when evaluating cancer outcomes. This information should be used in the public health assessment to (1) qualitatively describe the cancer-causing potential of a particular substance, and (2) compare site-specific dose estimates with doses or exposure concentrations shown to result in cancer in experimental studies or epidemiologic studies. This process is aimed at weighing the available evidence—in light of uncertainties—and offering perspective on the plausibility of cancer outcomes under site-specific exposure conditions.
-
Cancer classification. When communicating the potential for cancer hazards, state how strongly associated a substance is with cancer outcomes. Various government agencies and organizations use a "weight-of-evidence" approach in evaluating substance-specific carcinogenicity. The U.S. Environmental Protection Agency (EPA), the National Toxicology Program (NTP), and the International Agency for Research on Cancer (IARC) classify carcinogens based on the strength of the scientific evidence linking the substance with cancer outcomes under the reported conditions of testing.
Discussions of carcinogens should therefore include these classifications. The most current cancer classification information can be obtained from ATSDR's comparison value tables, which are updated quarterly. More detailed information on the carcinogen classification for a specific substance can obtained through EPA, IARC, or NTP.
When discussing a chemical's carcinogenicity, explain in plain language what the different classification categories mean. For example, "human studies clearly link the substance of interest with certain cancers" or "while some animal studies have shown increased tumors after exposure to the substance of interest, human data do not suggest a link between the substance and cancer in humans." Note that ATSDR evaluates the relevance of animal data to humans on a case-by-case basis. In the absence of compelling data to the contrary, however, a substance that has been shown to cause cancer in animals is considered to be carcinogenic in humans.
-
Identifying effect levels or a point of departure. For known or potential human carcinogens, understanding the doses at which cancer effects might be expected under site-specific exposure conditions requires an understanding of the dose-response curve for the substance of interest. Most available toxicologic data report cancer effects at doses much higher than those likely to be seen at hazardous waste sites. A first step therefore is to look at dose levels in this range of observation. In some cases (similar to the benchmark dose described above), toxicologists model available dose-response data to identify a "point of departure" (or an estimated or modeled dose that is near the lower end of the observed range). For example, a 5 or 10 percent effect level is often selected as the point of departure. This point of departure is then used as a stepping-off point for evaluating possible cancer effects at lower doses.
As stated previously, various mathematical models have been developed to predict the potency of substances at low doses. These models are based on scientists' understanding of the slope of the dose-response curve at high doses, and a series of assumptions about substance-specific behavior at doses below the range of observation (e.g., below the point of departure). When applying these models, scientists have by default historically assumed no threshold (or linear dose-response). As scientists learn more about the mechanism or mode of action by which carcinogens act, they are learning that this might not always be the case (EPA 2003a; Bogdanffy et al. 2001).(4)
Health assessors are not expected to conduct the types of modeling analyses described above. However, considering the following questions will help the assessor understand the behavior of a particular carcinogen. This perspective is then communicated in the public health assessment document.
- At what levels have cancer effects been reported in the literature? Proceed with caution, but comparing site-specific doses with the lowest reported cancer effect levels (CELs) can offer some perspective. Realize that CELs presented in the toxicological profiles represent only a snapshot of observed effect levels. As discussed above, it is not known whether lower doses will elicit a carcinogenic response. Also, review EPA's IRIS summaries and toxicological reviews to understand the basis for EPA's cancer slope factors and the studies used to support risk assessment decisions, including identified effect levels or calculated points of departure.
-
What is known about a substance's mode of action that might increase or decrease the likelihood of a cancer response at low doses? As emphasized in EPA's guidelines for cancer risk assessment, knowing the manner in which cancer is initiated or promoted by a substance (i.e., the mode of action) will help in determining the following: (1) whether a "safe" level or threshold may exist for that particular substance, or (2) whether evidence or sufficient uncertainty exists to suggest that even at very low doses cancer potential cannot be ruled out (EPA 2003a).
In cases where low dose extrapolations have been used to quantify a theoretical estimate of cancer risk, it is critical to put the calculated risk into perspective when discussing site-specific cancer hazards. Remember that any such estimate is based on several conservative assumptions to account for uncertainties. The true risk might be much lower; it might even be as low as zero (ATSDR 1993; EPA 2003a). Therefore, the health assessment team is encouraged to compare site doses with observed effect levels reported in the toxicologic and epidemiologic literature and discuss those site doses qualitatively in the context of issues presented throughout the remainder of this chapter.
- At what levels have cancer effects been reported in the literature? Proceed with caution, but comparing site-specific doses with the lowest reported cancer effect levels (CELs) can offer some perspective. Realize that CELs presented in the toxicological profiles represent only a snapshot of observed effect levels. As discussed above, it is not known whether lower doses will elicit a carcinogenic response. Also, review EPA's IRIS summaries and toxicological reviews to understand the basis for EPA's cancer slope factors and the studies used to support risk assessment decisions, including identified effect levels or calculated points of departure.
Evaluating carcinogens in this manner—assuming scientific data are available to support the analysis—provide the type of information needed to better communicate hazard potential to the community. A balanced discussion of what is known and not known will help provide more meaningful perspective to the community.
As our understanding of substance-specific toxic action grows, public health conclusions can change. Toxicologists at ATSDR and at other agencies, such as EPA, are reviewing available toxicologic information on an ongoing basis to help ensure the most accurate and scientifically defensible assessment of substance-specific hazards. The examples below illustrate the potential significance of identifying, understanding, and communicating the current understanding of a substance's toxic action.
In examining tumor responses in mice exposed to chloroform, scientists have discovered that chloroform appears to work through a non-genotoxic mode of action—that is, tumor responses are produced only at dose levels that result in cytotoxicity. Therefore, NOAELs have been identified both via ingestion and via inhalation routes of exposure below which no increases in cancer would be expected (Jorgenson et al. 1985; Larson et al. 1994 and 1996). As a result of these studies, EPA has determined that the oral reference dose (for non-cancer effects) for chloroform is protective against an increased risk of cancer, and EPA is currently working to revise its assessment for inhalation exposure (EPA 2001).
Using the newer inhalation data instead of the default linear dose extrapolation method could result in marked increases in predicted "safe" exposure concentrations. Based on this newer understanding, Larson et al. (1996) contrast a safe exposure concentration of 0.01 parts per million (ppm) of chloroform in air to the current IRIS value of 0.000008 ppm, even after applying an uncertainty factor of 1,000.
On the other hand, remaining uncertainties related to arsenic behavior at low doses have prompted regulators to lower the drinking water standard for arsenic.
|
What if no health guidelines exist? For some substances, no health guideline has been derived. This could be due to inadequacies and uncertainties in the available scientific literature. In such cases, consult with the toxicologist on your team to review the most current dose-response data and the status of any pertinent research. If appropriate study data can be identified, draw inferences using the guidance provided in the remainder of this chapter. If no or limited data are identified, review exposure potential and determine whether the absence of toxicity data is considered a critical information gap to assessing possible site hazards. If so, the team might recommend the need for further research (see Chapter 9). Remember, the narrative of the PHA should clearly state what is known and what is unknown about the toxicity of the substance in question. You need to explain clearly and justify your conclusions and recommendations. |
8.3.2 Assess the Relevance of the Critical Study
Whenever reviewing dose-response data, an understanding of the underlying study is pivotal. If the dose comparisons discussed above reveal the need for further analysis, judging the relevance of the critical study used in developing a health guideline to the site-specific exposure situation will provide another piece of information to guide health conclusions. (These factors are relevant when reviewing other studies as well). As the health assessor, you will add site-specific knowledge and insight that will be critical to this evaluation.
You should be able to perform the basic steps of a data review. Assessing the relevance of available studies requires both technical expertise and professional judgment. Numerous considerations beyond the scope of this guidance manual affect the quality of experimental data and its relevance to site-specific exposures. Most relate to experimental design. This list, and associated examples, should not be viewed as a complete guide for evaluating all toxicologic studies but as a general guide to aid you in the context of the public health assessment process. Again, work with the appropriate experts on your team when evaluating the importance and implications of such questions. In collaboration with the toxicologist and epidemiologist on your team, consider the following types of questions when evaluating how study features might make harmful effects more or less plausible.
-
Is the critical study based on human or animal data?
Clearly, a study based on human data holds the greatest weight in describing relationships between a particular exposure and a human health effect. Fewer uncertainties exist about potential outcomes documented in well-designed epidemiologic studies.
Exceeding a guideline value based on human data provides relatively strong evidence for the potential for harmful effects. Similarly, falling below a NOAEL reported in a human study could provide support for a conclusion that adverse effects are unlikely. However, before making this determination, the health assessor should consider the quality of the study and the size of the exposed group. Similarities and differences between available study data and your site-specific exposure conditions (e.g., exposure route, chemical form) should also be considered. -
How relevant is the dosing method to site exposures?
The relevance of the findings of an experimental study to environmental exposures will be influenced by how the test animal received its dose (e.g., gavage/water, gavage/oil, water, food, or vapor). Often, the exposure route in experimental studies is different from the route by which people living near a site could be exposed. Identify and discuss the differences to provide the reader with a sense of how differences can influence the likelihood of adverse health effects.
For example, a laboratory study in which animals were administered a substance via gavage or drinking water might not directly apply to a soil-exposure scenario. This is because solubility is often an important component of how much and how quickly substances are absorbed, which might impact the nature of the toxic response. The form of the substance tested in water and gavage can differ considerably from the form present in soil. For similar reasons, a dietary animal study might not adequately represent exposures from drinking water.
As another example, pregnant rats gavaged with oil solutions of trichloroethylene (TCE) might be consuming much more TCE per dosing than pregnant women drinking from TCE-contaminated wells. The dose received by pregnant rats in oil could far exceed the dose even possible in drinking water because of differences in the solubility of TCE in oil as compared to water.
-
How might dosing regimens influence the interpretation of the study data?<
In addition to the method of dosing described above, the dosing regimen can influence the absorption and ultimately the effects observed in experimental studies. You will want to examine how closely, in relative terms, the study conditions match site-specific exposure conditions. Some questions to ask include: Were animals dosed continuously or intermittently? Were animals dosed over the short or long term?
For example, the same dose administered in the shorter term (e.g., 28 days) might produce different effects than those produced after a longer-term dose administration (e.g., 90 days). Because different dosing regimens can produce different effects or affect the severity of the observed effect, one can be more confident the more closely study data match site-specific conditions. If only acute or subchronic dose data are available, state the uncertainties of applying such data to longer-term exposures. -
Is the form of the toxicant in the selected study the same or different from the form detected at the site?
The form or valence state of a substance can affect its bioavailability, its distribution within the body, and ultimately its toxicity. If study data are not available for the form of the substance present at your site, determine and explain in the PHA whether the chemical form at your site could be more or less bioavailable, or more or less toxic, than the form used in the study.
For example, the oral intermediate MRL for uranium is derived from a drinking water study. This is an important consideration when estimating doses for the soil ingestion pathway. A review of human data indicates that the fractional absorption of soluble uranium compounds is an order of magnitude greater than that of insoluble uranium compounds (ATSDR 1999a). In weathered soils, insoluble uranium compounds will predominate. Therefore, using the MRL to assess exposure to uranium in soil would be overprotective, because of the reduced bioavailability of uranium in soil as compared to water.
As another example, most arsenic in fish is in an essentially non-toxic organic form known as arsenobetaine (fish arsenic). Inorganic arsenic, which is considerably more toxic, makes up only a small amount (1–20%) of total arsenic in fish (ATSDR 2000; Francesconi and Edmonds 1997; FDA 1993). Therefore, if you were evaluating arsenic exposures via fish ingestion, you would need to account for this factor.
-
Are the effects observed in animals expected in humans?
If dose levels from animal studies (e.g., in mice, rats, monkeys) are being used to evaluate site exposures, determine whether any human or any in vitro studies are available that suggest a similar effect in humans. In addition, metabolism or mechanistic data, if available, could provide insight as to whether observed effects might be unique to, or different in, the study animal as compared to humans. If such data do not exist, assume that similar effects would occur in humans.
Some possible scenarios include: the metabolism of a chemical in animals could produce more or less toxic intermediates than in humans; the metabolism in humans could occur by another pathway and produce more toxic, non-toxic, or less toxic intermediates; or toxic intermediates could be produced at the high levels of exposures administered in the animal studies, but not at lower exposure levels. (See also discussion on toxicokinetics and mechanistic data in Sections 8.5.1 and 8.5.2, respectively). -
How relevant are observed health endpoints to specific community health concerns?
While health-based guidelines are typically designed to be protective of the most sensitive effect, it is important to familiarize yourself with the range of effects associated with a given chemical in the dose range of concern. This could provide added perspective as well as help in addressing community health concerns.
For example, if an MRL is based on increased kidney weight in rodents and the community is concerned primarily about blood-related disorders, you might want to look beyond the critical study for substance-specific data related to hematologic effects following exposure to the substance of concern (see Section 8.4). -
Does the bioavailability of the substance differ in the study matrix versus the environmental matrix being evaluated?
The bioavailability of a contaminant depends on its chemical properties as well as properties of the matrix. The bioavailability of a substance influences how much is absorbed by the human body and ultimately the potential for harmful effects. Bioavailability should be factored into the analysis when there is evidence that the chemical form at the site is more or less bioavailable than is the chemical form used in the studies being used for comparison purposes. The bioavailability of a compound is discussed in toxicokinetics section of the toxicological profile.
Substances in solid matrices (e.g., soil) might be less well absorbed while passing through the digestive tract than would the same substances in water. This could be due to the solubility of the substance and the property of the matrix. Some forms of a salt can bind tightly to soil, thereby reducing its bioavailability. For instance, some forms of arsenic bind tightly to soil and are therefore not readily absorbed in the human digestive system. On the other hand, the same form of arsenic in drinking water can be released from the matrix and more readily absorbed (Alexander 2000). Ultimately, the rate of substance dissolution will determine its uptake and availability (Hardman et al. 1995). -
What uncertainties/limitations exist?
Identify any problems or limitations with the studies used to support your analysis. In most cases, uncertainties and limitations will be discussed in the Health Effects section of the toxicological profiles and in the discussion of the MRL derivation. IRIS summaries also discuss uncertainties and confidence in the critical studies evaluated by EPA.
The PHA should describe any limitations, uncertainties, and data gaps found in the available literature. Describe in qualitative terms the uncertainty factors used in the development of health guidelines. Also discuss the level of confidence in the studies as well as their overall applicability to site-specific exposures. The higher the confidence or level of certainty, the more weight the study will hold in your analysis.
8.4 Reviewing Other Dose-Response Data
As previously discussed, health guidelines are generally based on the lowest observed adverse effect levels reported in the literature, very often from a single study. In addition to the critical study, other studies can provide substance-specific, dose-response data. For substances of potential concern at a given site, the health assessor would never be expected to perform an exhaustive review of these studies. However, reviewing the larger toxicologic and epidemiologic database (e.g., the levels of significant exposure summarized in the toxicological profiles) provides additional supporting evidence for public health assessment discussions.
In the in-depth analysis, one looks beyond single points on the dose-response curve to gain a fuller understanding of the range of effects and effect levels observed in experimental studies. Both the shape and slope of the dose-response curve can help explain where site-specific exposures lie in the larger scheme of things. This will often help provide the perspective community members seek, and it will help you decide which, if any, harmful effects might be possible. In some cases, consistent findings might be seen across studies. For other substances, findings might be more disparate.
The most important thing for the health assessor to keep in mind is how to describe in plain language what is known and not known about the toxicity of a particular substance. Questions to consider include:
-
Where does the NOAEL or LOAEL for the critical study fall in relation to other studies? Although the critical study will weigh most heavily in your analysis, it might be helpful to describe the similarity or disparity of dose levels and health endpoints observed across studies. Your PHA should introduce information that will further support your discussion and eventual conclusions. For example, many of the reported effect levels in other studies for the substance of interest may be in the in the same general range as the critical study, strengthening the evidence that effects might be seen in that dose range.
Recognize the importance of not taking dose-response data at face value. The criteria described in Section 8.3.2 should be considered carefully. Remember, the critical study has been identified—after careful review of the scientific literature—as the best for developing protective health guidelines. The purpose of this exercise is not to discredit that effort, but to encourage consideration of the bigger picture.
-
If the health guideline is based on animal data, do any human data exist that shed more light on the issue? If extensive epidemiologic data are available for a particular substance, these data will likely have been reviewed and considered in the derivation of the health guideline for that substance (e.g., the MRL for methyl mercury). However, as a minimum, available epidemiologic data can be used to augment the findings of animal studies. For example, an occupational study can show that exposure to a particular substance is associated with the same toxic endpoint seen in animal studies. This observed species concordance would provide greater weight to the available animal dose-response data used to evaluate human health effects.
The exposure levels and associated outcomes, when available, can sometimes be used for comparison purposes with site exposures. For example, take the following scenario: Community members are concerned about low levels (2 parts per billion [ppb]) of a particular contaminant in drinking water that they have been drinking for approximately 10 years. They believe leukemia rates are elevated. Two independent studies of community drinking water supplies with 100 ppb of the same contaminant revealed no elevated leukemia or any other cancers in populations exposed for 30 years. In this case, the epidemiologic data might provide evidence supporting the fact that site exposures are unlikely to produce cancer effects at site exposure levels, notwithstanding possible study shortcomings. Furthermore, an understanding of toxicologic and epidemiologic data can help determine the biologic plausibility of a particular health outcome. Note that, depending on the community concern and other factors, an evaluation of health outcome data can be considered in such a case (see Section 8.6.1).
Because of the inherent limitations and uncertainties associated with environmental epidemiologic evaluations (generally due to the lack of adequate exposure data or sample size), epidemiologic data described in a toxicological profile or other sources should be used with caution. The health assessor should therefore call upon an epidemiologist to assist in evaluating the applicability and usability of literature-based or site-specific epidemiologic data.
Criteria have been established to guide epidemiologists in evaluating the strength of human data and should be kept in mind when you review and communicate such data in the context of your site-specific data (see text box below).
|
Evaluating Epidemiologic Studies Understanding the strengths and weaknesses of the various types of epidemiologic studies (e.g., occupational studies, community-based studies) will help determine the suitability of a particular study in supporting and drawing public health conclusions. For studies presented in toxicological profiles, these points are generally highlighted. The factors which experienced epidemiologists generally consider when reviewing the quality and overall utility of studies include:
In addition, a number of criteria assist epidemiologists in judging the causal significance of associations revealed in studies. These criteria, presented below, can help guide you as you evaluate and explain potential or dismissed causal relationships in your public health assessment. You can use these concepts in describing the evidence that a study(ies) might or might not provide—that is, the strength of the evidence linking a particular substance with a particular health outcome of interest. Individual criteria, if met, can support a causal relationship but can not prove it. The more criteria that are met, the more likely it is that an observed health effect is causally related to the exposure under study. Because the characterization of exposure is the weakest link in most epidemiologic studies, it will likely be the greatest limiting factor in establishing a causal relationship.
|
8.5 Evaluating Other Substance-specific Factors that Can Increase or Decrease the Potential for Harm
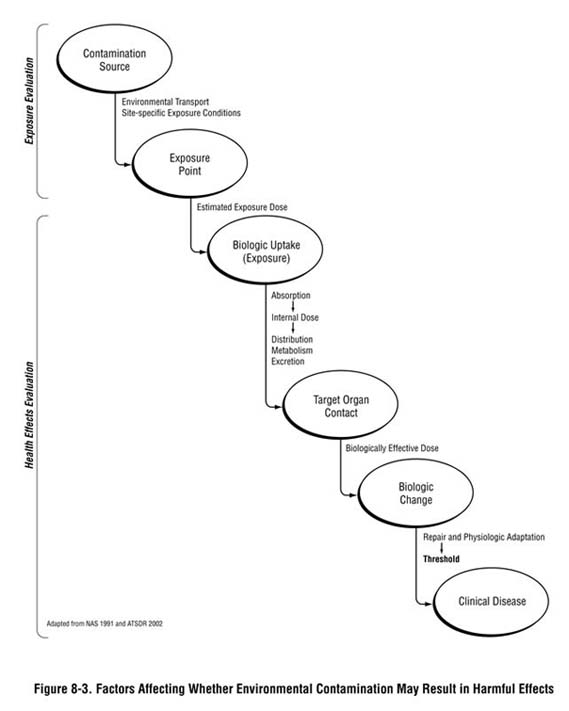
As depicted in Figure 8-3, multiple factors—other than the detected environmental concentration or exposure dose—influence whether an exposure could result in harmful health effects and what the type and severity of those health effects will be. A substance will only produce adverse or toxic effects if it or its metabolites reach specific sites in the body at a concentration and over a duration sufficient to produce an adverse effect. Whether exposure could lead to an adverse health outcome depends on the duration and characteristics of exposure and on the characteristics of the receptor population (e.g., developmental stage, existing disease state, genetics) that could make them more or less susceptible to site-related exposures. These factors are generally considered in the development of health guidelines and during the screening analysis, but might need to be examined more closely at this stage of the public health assessment process and described in your PHA narrative.
This section provides a brief overview of these factors and how they could weigh into your public health conclusions.
Substance-specific toxicokinetic or pharmacokinetic properties (e.g., absorption, distribution, metabolism, and elimination) largely influence whether a substance will reach a target organ and produce a toxic effect. If available, such information can be obtained from the toxicological profile or other data sources for toxicokinetic summaries. Determine what is known and not known about the extent to which a substance is absorbed. Also, how it is distributed through the bloodstream, changed to different forms, excreted, or ultimately delivered to target organs. When available, toxicokinetic data can be used in various ways to support your health effects evaluation. For example, it can used in interpreting the relevance of animal studies to human exposures—that is, by determining whether any distinct differences between animals and humans have been documented. For example, does the metabolism of the substance in animals produce more or less toxic intermediates than in humans? Is the substance absorbed more or less in animals compared to humans? Note that, in absence of data to the contrary, bioavailability is assumed to be the same in animals and humans under similar exposure conditions. For some substances, quantitative data can allow you to compare the bioavailability of a substance in experimental animals and humans.
Knowledge continues to grow on how various toxic substances produce biologic changes and the significance of those changes. In fact, this growing knowledge is modifying how human health hazards are assessed.
While this type of analysis is best left to the toxicologists, reviewing documentation (e.g., toxicological profiles, IRIS) on the nature of biologic changes triggered by a particular substance can be helpful in evaluating the behavior of that substance at low doses. Further, understanding the basic or specific biologic changes that ultimately lead to clinical disease in a test animal can aid in determining how well animal models might predict the same type of adverse effect in humans. A toxicologist might ask, for example, if the animal mode of action is plausible in humans, taking into consideration the kinetic and dynamic factors discussed above.
For a limited number of chemicals where biologic uptake and mode of action have been well studied and defined, physiologically based pharmacokinetic (PBPK) models have been developed to estimate dose levels in various body compartments and organs (e.g., lead). These models involve a series of mathematical equations that describe the pharmacokinetics of a chemical. Inputs into the models include the exposure dose and model parameters, such as tissue volumes, blood flow rates, partition coefficients, and metabolic rates. The output is the predicted internal dose (or target tissue dose). PBPK models are also beginning to be used to evaluate chemical mixtures (ATSDR 2001a; Krishnan et al. 2002).
Pharmacodynamic (PD) models are also available; these mathematical models describe the quantitative relationship between the target tissue dose and cellular and molecular changes associated with adverse health effects. Increasingly, PD models account for damage, repair, and compensation, and predict dose-response over a range of doses, both within and between species.
When PBPK or PD models are available and are applied, they can help to reduce the uncertainty in the health evaluation. Also, the models eliminate the need for cross-species extrapolation because they can account for differences in rates of biologic processes. For some substances, toxicologists have used such models in deriving health guideline values. The data used to support the model (e.g., metabolism and distribution data) can provide added perspective of how closely linked a particular dose might be to an adverse health effect. Although health assessors would not be expected to apply all of these types of models, an understanding of the general underlying principles can support the site-specific analysis, as described above.
Models also exist to estimate the radiation dose to specific organs and tissues, as well as total body dose. Like chemical exposures, ionizing radiation can produce many different effects, depending on (1) the type of radiation, (2) the radionuclide and its metabolic products, and (3) the dose received by the critical or most sensitive organ. When evaluating exposures to radiological contamination, enlist the help of a health physicist. See text box below for special considerations for radiological contaminants.
|
Radiation Exposures People can be exposed to radiation either externally or internally. External exposure occurs when a person is exposed to a source of penetrating radiation (beta particles of specific energies and gamma radiation) outside the body. Internal exposure occurs when radioactive materials are inhaled, are ingested, are absorbed through the skin, or taken in through wounds. The potential for health effects depends in part on the radiation dose delivered, the rate of delivery, and where in the body particular radionuclides concentrate. All radionuclides are partly absorbed from the lungs and intestinal tract into the bloodstream. From there they circulate throughout the body and either redeposit in other organs or are cleared by the kidneys for urinary excretion. In general, most radionuclides taken into the body by ingestion are excreted in the feces. Some radionuclides accumulate in specific tissues when taken internally, in the same manner as their non-radioactive forms. In general, the cells of the body that are most sensitive to ionizing radiation are those that have the most rapid rate of cell division. The cells in the body that are most sensitive to radiation are the progenitors of the blood cells, followed by the cells lining the gastrointestinal system (Hall 2000). The reader is cautioned, however, that effects resulting from radiation exposure can be difficult to ascertain. Proper knowledge of radiological dose assessment is essential before conducting a health assessment. Again, it is strongly recommended that a health physicist be consulted on these matters. In addition, the health assessor should recognize that radionuclides can pose significant chemical toxicity that is not related to their radiotoxicity. For example, the most sensitive health endpoint for exposure to uranium is its chemical toxicity to the kidney rather than its radiotoxicity. |
8.5.3 Sensitive Populations and Life Stages
Some substances have been shown to cause greater harm in particular populations or when exposure occurs at a particular point in life (e.g., fetal development). It is ATSDR policy that children's health issues must be considered at all sites (ATSDR 1998).
It is important to remember that sensitive populations are considered when MRLs and other health-based comparison values are developed. An uncertainty factor (e.g., a factor of 10) is generally applied to help ensure sensitive populations are amply protected. In addition, comparison values are developed to specifically account for children's exposures. Identifying or accounting for potentially sensitive or more highly vulnerable populations should also be a key component of your exposure pathway analysis (Chapter 6) as you estimate site-specific doses (Chapter 7). Thus when comparison values are not exceeded, health assessors can be confident that it is highly unlikely that even the most sensitive populations would be adversely affected.
However, when site-specific doses exceed comparison values, site- and substance-specific factors should be re-examined to evaluate to what extent, if any, a particular population is at increased risk of harm. Information on potentially sensitive populations can be found in the toxicological profiles in the section titled, Populations that are Unusually Susceptible. As stated, ATSDR places particular emphasis on children as a potentially sensitive population. For information on children's susceptibility, the health assessor should read those sections of the toxicological profiles that specifically discuss the susceptibility and exposure of children to chemicals (Children's Susceptibility and Exposures of Children).
Characteristics of certain populations might make them more sensitive to environmental exposures — because of underlying disease, other physiologic factors, or non-site related exposures. Many of these issues should be first addressed and highlighted during the exposure pathway and screening analyses (see Chapters 6 and 7). At this point in the analysis, you need to determine whether special characteristics of the substance and of the site community might affect public health conclusions.
-
Age. Children differ from adults in their exposures and can differ in their susceptibility to certain hazardous substances. Understanding when exposures occurred during critical periods of development is therefore important. The box below highlights some special considerations when evaluating children's health issues. Note that ATSDR and others continue to research the significance of early-life exposures to toxic substances, both for cancer and non-cancer outcomes. Much of the impetus for such an approach is the growing knowledge and understanding of how a substance exerts its effect (i.e., its mode of action) and how, if exposure occurs during early-life stages, a particular mode of action can increase the risk of a toxic response (EPA 2003b).
The literature suggests that elderly populations may have significantly heightened susceptibility to some contaminants because of lower functional capacities of various organ systems, reduced capacity to metabolize foreign compounds, and diminished detoxification mechanisms. It is difficult to generalize, however, due to variations across individuals and different rates in biological system breakdown (Hardman et al. 1995). Another important consideration is that older individuals may have much different exposures than younger adults and children.
- Sex. Some substance-specific adverse health effects can be mediated by hormonal influences and other factors that are sex-linked. In general, sex-linked differences in toxic susceptibilities have not been extensively investigated. However, it is well documented that, because of various physiologic modifications in the body that occur during pregnancy, pregnant women are often at significantly greater risk from exposure to beryllium, cadmium, lead, manganese, and organophosphate insecticides than areother members of the general population(Calabrese 1986).
- Genetic background or ethnicity. Some research suggests that certain genetic factors can increase the risk of developing chemically-induced health effects, though further research is needed (Calabrese 1994). Factors that can affect the susceptibility of exposed groups include acetylation phenotype (i.e., fast versus slow acetylators), sickle cell trait, and glucose-6-phosphate (G6PD) deficiency (Rios et al. 1993). In addition, individual variability in the induction of metabolic enzymes could cause people to respond differently to the same environmental exposure. For health assessment purposes, the susceptibility of the most sensitive subgroups should be considered.
- Health and nutritional status. Understanding the location and characteristics of subgroups, such as the elderly and those of lower socioeconomic status, will help identify pre-existing health conditions (e.g., asthma, nutritional deficiencies) that might influence the impact of site exposures. Locations of schools, playgrounds, recreational areas, retirement homes, or convalescent homes on or near a site should be carefully noted as important indicators of the presence of potentially sensitive populations.
- Cultural practices. Various practices (e.g., ceremonies among American Indian and Alaska Native populations, subsistence fishing, medicinal use of plants) can lead to increased exposures. These factors should be considered as part of your exposure assessment and when estimating site-specific exposure doses (see Chapters 6 and 7).
|
Special Considerations Related to Child Health Per ATSDR policy, children's health issues must be considered at all sites (ATSDR 1998). ATSDR recognizes that developing fetuses, infants, and children can be more sensitive to exposures than are adults in communities faced with contamination of water, soil, air, or food. That is why where possible, ATSDR develops health guidelines to account for possible adverse health effects in children. Identifying possible site-specific exposures to children is a critical step in your exposure evaluation (see Chapter 6). As with adults, when site-specific doses exceed health guidelines for children, a closer examination is necessary. When evaluating the possible public health significance of child exposures at your site, consult the toxicological profile or other data sources to identify substance-specific data that might indicate a higher or lower likelihood of harmful effects in children. Consider the following types of questions (ATSDR 1999b).
For more background information on the important topic of children's health, see the references and resources listed at the end of this chapter. Also consult with the toxicologist on your team. |
8.5.4 Multiple Chemical Exposures
The approaches outlined in this manual focus largely on evaluating chemical-specific and pathway-specific exposures. That is, health effects are examined for individual chemicals for specific exposure pathways (e.g., ingesting benzene in drinking water). In reality, exposures can involve multiple chemicals and can occur through more than one exposure pathway. Approaches for evaluating the effect of multiple pathways are discussed in Chapter 7. This section highlights how to approach multiple-chemical scenarios.
The health impact of exposure to chemical mixtures can be of particular concern at hazardous waste sites, since most contain multiple chemical contaminants. While in many cases it might suffice to evaluate exposures on a chemical-by-chemical basis, in some cases you might need to examine the combined action of chemicals (e.g., additive, antagonistic, synergistic, and other interactive effects).
A first step in understanding the potential significance of multiple chemical exposures is to read the Interactions with Other Chemicals section of the toxicological profile about any known interactions among the substances detected at your site. These profiles can provide insight regarding what is known and what is not known about interactions among various pollutants. For many chemicals, however, information on toxic interactions is lacking, and the available literature focuses on the effects of chemical interactions at exposure doses that are much higher than those that are typically encountered at hazardous waste sites. Furthermore, even though limited information for some chemical mixtures is available, no empirical data set could account for the infinite array of chemicals in varying proportions that can be found at sites.
When conducting public health assessments, it is particularly important to understand potential toxic interactions at environmentally relevant doses of chemicals. However, relatively few studies have been conducted to assess toxic interactions in these low dose ranges. A series of important studies on the toxicity of low dose chemical mixtures was conducted by the TNO Nutritional and Food Research Institute in the Netherlands (Jonker et al. 1990; Jonker et al. 1993). In these experiments, rats were dosed with mixtures of chemicals at doses near their individual NOAELs and LOAELs. The results of these experiments indicated that there was no discernable toxic response until the dose levels of the individual chemicals approached or exceeded their individual thresholds. However, when the chemicals were administered at their individual LOAEL doses, there was clear evidence of additive toxic effects. Furthermore, additive toxicity was observed even though the chemicals had different mechanisms of toxicity.
Other studies have provided evidence that exposure to chemical mixtures, in which the chemicals were administered at doses that were near their individual thresholds, can produce additive toxic effects. For example, rats exposed to a mixture of subthreshold doses of 1,1,1-trichloroethane, trichlorethylene, and tetrachloroethylene experienced signs of liver toxicity (Stacey 1989). In an oral feeding study, rats were dosed with cadmium and lead. Neither metal, by itself, significantly affected hemoglobin or hematocrit levels; but when the metals were administered as a mixture, significant decreases in these parameters were observed (Mahaffey and Fowler 1977).
However, there is no evidence of additive toxicity from exposure to chemical mixtures when the individual chemicals are administered at doses that are well below their individual thresholds (Seed et al. 1995; Wade et al. 2002). Nevertheless, the threshold doses for many toxic endpoints in animals are not well defined. Therefore, it is prudent for the health assessor to consider the potential for toxic effects from exposure to chemical mixtures at all sites. Inthe health assessment, the assessor should indicate that he has evaluated exposures to chemical mixtures and considered the potential for chemical mixture interactions.
As part of this evaluation, the health assessor should calculate a Hazard Index (HI) for the mixture of chemicals at a site. A HI is defined as the sum of the quotients of the estimated dose of a chemical divided by its MRL or comparable value. In mathematical terms,
| HI | = | Dose1 MRL1 |
+ | Dose2 MRL2 |
+ | Dose3 MRL3 |
+ | . . . . . | Dosen MRLn |
For additional information on calculating an HI, see ATSDR's Guidance Manual for the Assessment of Joint Action of Chemical Mixtures. This manual is available on CD-ROM and on the ATSDR Web site.
If the HI is less than 1.0, it is highly unlikely that significant additive or toxic interactions would occur, so no further evaluation is necessary. If the HI is greater than 1.0, then further evaluation is necessary as described below.
For chemical mixtures with a HI greater than 1.0, the assessor should compare the estimated doses of the individual chemicals to their NOAELs or comparable values. If the dose of one or more of the individual chemicals is within one order of magnitude of its respective NOAEL (0.1 x NOAEL), then there is a potential for additive or interactive effects. Under such circumstances, the assessor should conduct an in-depth mixtures evaluation as described in ATSDR's Guidance Manual for the Assessment of Joint Action of Chemical Mixtures.
If the estimated doses of the individual chemicals are less than one-tenth of their respective NOAELs, then significant additive or interactive effects are unlikely, and no further evaluation is necessary. In some instances, however, the assessor might choose to evaluate further the potential for additive or interactive effects because the chemicals in the mixture have the same target organ, have the same mechanism of action, or for other reasons. In these instances, the assessor can conduct an in-depth quantitative mixtures analysis as described above.
Another valuable resource for information on chemical mixtures is the Interaction Profiles for priority chemical mixtures. ATSDR is developing these profiles for chemical mixtures that are of special concern to ATSDR, such as Persistent Chemicals Found in Fish (ATSDR 2002). These documents use a weight-of-evidence approach to evaluate the influence of interactions in the overall toxicity of the mixture. The documents also develop target organ doses that can be used to evaluate the impact of the chemical mixture on different target organs.
8.6 Evaluating Site-specific Health Effects Data
Another line of evidence that can provide additional site-specific perspective is the availability of meaningful health outcome data or human exposure data. In certain cases, data from health outcome data evaluations can provide evidence—ranging from weak to strong—of plausible associations between substance- or site-specific exposures and human health effects. In some cases, biologic data (e.g., site-specific substance concentrations in blood or urine) collected as part of exposure investigations, might be available and offer some insight on the extent of actual exposure (beyond the exposure-dose estimates generated from environmental concentrations). In rare cases, individual medical reports might be available, documenting symptoms or the results of clinical examinations. Note, however, that in most cases there is a lack of data to correlate biologic levels with health effect levels. This section describes how to determine whether such data can help support your public health conclusions.
This section provides guidance to health assessors for addressing health outcome data in the public health assessment process. Health outcome data are existing data that measure disease mortality or morbidity. Health outcome data analyses or reviews are descriptive epidemiologic analyses.
In all public health assessments, ATSDR is required by the Superfund law to consider the evaluation of mortality and morbidity data (e.g., health outcome data). The law indicates that a public health assessment should include relevant health outcome data analyses when exposure to site contaminants could have resulted in the development or exacerbation of health effects. The guidance presented below reflects the deliberations of the ATSDR Work Group, whose members examined the decision criteria used to evaluate the appropriate use of health outcome data in the public health assessment process.
Decisions about how to use or analyze health outcome data—or whether to use it at all—should be made with the assistance of various disciplines. To reach a prudent decision, a health assessor might include input from epidemiologists, statisticians, toxicologists, community involvement specialists, health educators, and environmental scientists such as engineers or geologists.
Inclusion of a health outcome data evaluation in a public health assessment can achieve the following if it is determined that it is appropriate to include such an evaluation:
- Comparison of the occurrence of disease between a population potentially exposed to site contaminants and an appropriate reference population, such as the county, the state, or the United States.
- Assistance in addressing community concerns about the occurrence of disease in potentially exposed individuals.
- Identification of the potential need for follow-up health actions such as exposure investigations, analytical epidemiologic studies, or health surveillance.
Traditionally, at the outset of the public health assessment process, the health assessor in concert with the site team gathers community concerns and informs community members about ATSDR products and services. During this period the site team should provide to community members information about the utility of analyzing health outcome data. Specifically, community members should be informed of how ATSDR uses health outcome data, when it is available, and the criteria and rationale used to determine whether a health outcome data evaluation would enhance the public health assessment decision-making process. Therefore, regardless of whether health outcome data are used in the public health assessment itself, the analysis of the criteria for each site, as described below, is in essence the first step in the evaluation of health outcome data.
The team should use the answers to the following questions as a guide in determining whether a public health assessment should include analysis and interpretation of site-related health outcome data. See also Figure 8-4.
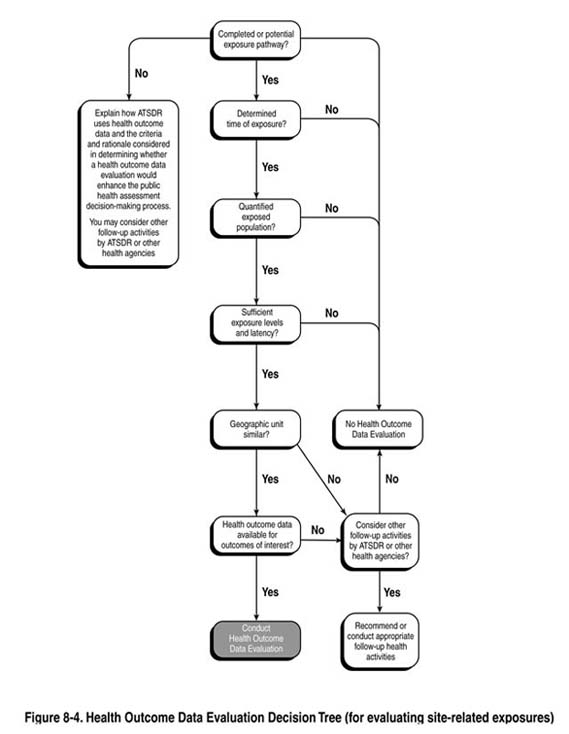
The criteria below focus on site-related exposure considerations only. Regardless of what path you follow, your PHA discussions must clearly describe the rationale for the decision, and how your exposure evaluation factored into the decision. In some cases, community concern about illness in their community could be a sufficient trigger to pursue health outcome data, even in the absence of a potential or completed exposure pathway. Assuming data are available for the disease(s) of concern and the geographic unit under evaluation, a health outcome data evaluation would determine whether disease rates are elevated in the community. While no possible site-specific link might exist, information regarding the presence or absence of elevated disease rates could either help allay fears or identify a disease trend in the community warranting follow-up.
The decision to proceed under such circumstances is left to the discretion of the site team, but is generally not considered part of the public health assessment process.
- Are there one or more current (or past) potential or completed exposure pathways at the site as defined in section 6.6? If there are none, conducting a health outcome data evaluation will not be helpful in assessing potential harm related to the site.
- Can you determine the time period of exposure? If the length of exposure and places where exposure did occur, or is occurring, cannot be estimated, the requirement to consider analysis of site-related health outcome data is complete.
A reasonable estimate of the length of exposure is necessary for determining whether the health outcomes evaluated are site-related. This ensures that the health outcome data being analyzed could be the result of exposure to site contaminants. The relevant exposures could have been for a few days or many years before the onset of disease, depending on the chemical involved, the age of the individual exposed, the specific health outcome, and other factors. The health assessor should ensure that the available health outcome data are from the time period when site-related health effects are likely.
- Can you quantify the population that was, or is, being exposed? The evaluation of possible links (or associations) between site-related exposures and illness or disease in a population is not scientifically reasonable unless a reliable estimate can be made of the number of people exposed and the total number of people in the study population. The availability of demographic information within the exposed and non-exposed study population (e.g., age, number of years at residence, smoking status) is also an important consideration. If such an estimate cannot be made, the requirement to consider analysis of site-related health outcome data is complete.
Statistics might be available showing the number of people identified with certain health outcomes in a selected population. However, an estimate of the number of people exposed is needed to calculate the rate of health outcomes among the exposed population. This information is required to adjust the mortality or morbidity (i.e., incidence/prevalence) rates in the exposed population to the population(s) used for comparison (i.e., non-exposed) to determine any difference in disease rates. To identify the exposed population, the health assessor needs information on where exposure occurred (i.e., geographic extent of exposure).
Analysis of health outcome data could be impractical in sparsely populated areas—the population is too small to measure the rate of a disease. For example, if the "expected" rate for a particular disease is 5 in 1,000,000 and the exposed population only numbers 100, the absence of this disease over a short time period in the exposed population in itself will not provide much perspective. Moreover, if the disease of interest is very rare it could require a large population or, at the very least, several years of mortality or incidence data to allow any useful interpretation. Alternatively, the presence of one or two cases of a rare disease in a small, exposed population does not automatically link the exposure to the disease. It is important to identify the time period in which the cases occurred and any known risk factors, other than the exposure, that could be present in the exposed population.
- Are the estimated exposure doses(s) and the duration of exposure sufficient for a plausible, reasonable expectation of health effects? Analysis of site-related health outcome data is not scientifically reasonable unless at least a qualitative estimate of exposure doses can be made. If such an estimate cannot be made, the requirement to consider analysis of site-related health outcome data is complete; no further analysis is appropriate.
Analysis should not be done if quantitative exposure data for the exposure period of interest and the exposure doses calculated from those data are below the no observed adverse health effect level (NOAEL), or if there is no NOAEL, the lowest observed adverse effect level (LOAEL) for the chemicals being evaluated.
Qualitative exposure estimates come from exposure scenarios in which strong circumstantial evidence suggests that exposure occurred for long enough and at a sufficient enough concentration for health effects to be possible. Such evidence could include monitoring data from nearby areas, violations of air-release or water-discharge permits, reports from residents, observations by the health assessor or other knowledgeable individuals, or other relevant information. Qualitative estimates should be based on more than one type of evidence and should be made in consultation with knowledgeable environmental staff.
- Are health outcome data available at a geographic level or with enough specificity (i.e., census tract or census block) to allow for correlation with the exposed population? To be able to analyze for health effects that might be site-related, the health assessor needs to be able to make an approximate identification of the exposed population within the data source or database to be utilized. If this is not possible, the requirement to consider analysis of site-related health outcome data is complete; no further analysis is appropriate.
To assess potential site-related effects, health assessors need to be able to separate the health outcomes for the exposed population from the unexposed population (at least as much as possible). If the area for which the disease rate can be calculated using the health outcome data is much larger than the area exposed, then exposure mis-classification bias will be introduced, and disease risks will be severely underestimated. For populations with past exposures, a site with high population turnover (in- and out-migration) could be the basis for not analyzing health outcome data because of the possibility of exposure mis-classification.
- Do the validated data sources or databases have information on the specific health outcomes or disease(s) of interest likely to occur from exposure to the site contaminants and are those data accessible? When analyzing health outcome data that could be site-related, the health assessor should focus on specific, sufficiently known (or suspected) health outcomes in the available morbidity or mortality databases (e.g., specific cancers, specific birth defects).
The health outcomes likely to occur from exposure to site contaminants might not be in the available databases. For example, if exposure to a contaminant is linked to birth defects but not to cancer, it is notappropriate to evaluate cancer data because they are available and birth defect data are not.
If a health outcome data analysis is performed, there should be a coordinated effort among all the staff involved with the site to inform or educate community members. Also, the community should be informed about the strengths and limitations of descriptive epidemiologic analyses.
In particular, the community must be made aware that descriptive epidemiologic analyses cannot establish cause and effect. Elevated disease rates alone cannot be considered conclusive evidence that living near a waste site is the sole cause for the occurrence of a specific disease. Health outcome or descriptive epidemiologic analyses are only an initial step in determining the nature and extent of disease in the community around a site, and what that might mean.
If it is decided that a health outcome data analysis should be included in the public health assessment, the team should seek assistance from an epidemiologist knowledgeable in analyzing mortality and morbidity data.
Every public health assessment should include a brief description of the requirement to consider health outcome (mortality and morbidity) data and the reasons why a health outcome data analysis was or was not included in the document. If a health outcome data analysis is included, then the public health assessment should have a concise description of the methodology used and the results and limitations of the analysis.
In some cases, biologic data might be available to further define or quantify exposures to site contaminants. Biologic data for exposed or potentially exposed populations can provide additional evidence when evaluating potential health effects. Depending on the levels detected, it could support or disprove plausible biologic outcomes. Site-specific, biologic sampling results must be interpreted with caution. Specifically, issues that you and the other experts on your team need to consider include:
- Biologic data, like environmental data, need to be collected by trained professionals and analyzed in a standard way.
- Detected levels might not be the result of site-related exposures (e.g., increased blood lead levels could be the result of lead paint exposures or traditional medicines).
- For chemicals with short biological half-lives, results will likely only represent recent exposures.
- The correlation between detected levels and clinical effects might not be understood.
- The people tested might not be representative of the exposed population (i.e., results from a small sample group may not reflect the range in exposures across the entire exposed population).
Biologic testing is most commonly conducted using blood or urine samples. However, background or reference ranges for many chemicals in blood or urine have not been well defined. The utility of hair analysis as a biomarker of exposure to environmental contaminants is not well established except for methylmercury (ATSDR 2001b; Harkins and Susten 2003).
When biomonitoring data are available, they can provide additional perspective for the health assessor. Measured levels can be compared to levels shown in the literature to be associated with overt clinical effects from case studies or more subtle effects that might be inferred from population-based studies. Useful information sources on biomarkers include ATSDR's toxicological profiles (sections related to biomarkers) and Case Studies in Environmental Medicine. In addition, human exposure data for selected environmental contaminants are being collected as part of the CDC's National Health and Nutrition Examination Survey (NHANES).
In 2003, the National Center for Environmental Health reported biomonitoring data for 116 environmental chemicals in the non-institutionalized, civilian U.S. population (CDC 2003). In the future, the list of chemicals will be expanded to include other important environmental contaminants. These data are valuable in comparing an individual's exposure to a chemical to exposure levels in the general U.S. population. However, these data only reflect national exposure levels, and they are not indicators of potential adverse health outcomes. Health assessors should consult with medical professionals and toxicologists for guidance in interpreting the health significance of biomonitoring data.
8.6.3 Medical Data and Information
Medical data, such as individual medical reports or logs of health conditions reported by community members, could be presented to ATSDR for evaluation in the public health assessment process. This type of data could provide some additional insights to health issues in the site community. But any form of medical data must be used and interpreted with caution. First, if the data are privileged or confidential, precautions must be taken to respect the individual's right of privacy (see Chapter 3, Section 3.5). Second, the documentation of a particular medical condition in an individual(s) does not inform you of causes or patterns of disease in the community. It is necessary to identify plausible biologic links between exposure and reported medical concerns. Credible reports of illness or disease, along with available health outcome data, can be used to support recommendations for public health actions, such as targeted biologic sampling or a health study.
8.7 Presenting Findings in the Public Health Assessment Document
As you and your team consider the topics highlighted in this chapter, you will face the challenge of integrating and communicating the findings of the analysis in a clear and concise way in the public health assessment document. As mentioned repeatedly, the goal of the in-depth analysis is to put site-specific exposures into perspective. This requires integrating the exposure and health effects data that have been identified throughout the public health assessment process and describing in qualitative terms those exposures most likely to be of public health concern and most likely to require public health action. As part of this process, you will probably need to integrate conclusions generated by a variety of analyses and, possibly, performed by various specialists.
The Discussion section of the public health assessment document should include narratives describing the exposures that could be of greatest concern. It should also state clearly those exposures that are not of public health concern. Keep the main discussions brief and include only information that will help the reader understand the public health conclusions. The focus should be on the possible health concerns of the potentially exposed populations. Do not present a mini-toxicological profile with information that has little relevance to the site or to the exposure situation under discussion (e.g., describing all physical characteristics of the chemical, all reported adverse effects, etc.). Include in-depth toxicologic evaluations and dose calculations in an appendix, as determined by the information needs of your audience.
Because sites differ, the emphasis of discussions can vary depending on site-specific conditions. No specific formula can evaluate the range of exposure conditions that might be observed across sites. No specific weighting factors can be assigned to each factor considered throughout the analysis. The process is one of judgment. Still, use of the guidance presented throughout this chapter and, when assimilating the findings of the analysis, consideration of the following questions will help ensure the scientific evidence is explained in a clear and consistent manner across sites. You will be building on information from other steps in the public health assessment process.
- What pieces of evidence were used in the analysis and why? Describe exposure conditions (see Chapters 5 , 6, and 7) and health effects data. Tie in exposure condition information that might provide additional perspective (e.g., how exposure levels compare to background and the likelihood of exposures). Explain all assumptions used to estimate site-specific doses (see Chapter 7). Identify the overall availability of pertinent health effects data for the substance and pathway of concern.
-
What information evaluated by the site team will help provide dose perspective? Describe how site-specific exposure levels compare to observed health effect levels reported in relevant studies. Consider possible acute and chronic adverse health effects. Where possible and appropriate, present ranges of effect levels reported in the literature.
Be as explicit as possible about why exposure levels are or are not expected to be a potential problem. For example, do not state that "groundwater contaminant exposure levels were too low to be of health concern." Instead, indicate that "exposures to detected groundwater contaminants are not expected to result in adverse health effects because dose estimates were 5,000 to 10,000 times lower than those shown to cause harmful effects in both human and experimental animal studies." Qualifying terms such as "low" or "high" by providing a comparison will help provide perspective. Also, state any assumptions used in your dose estimates (e.g., ingestion rates, exposure duration).
- What site- or substance-specific factors might affect the ultimate toxic potential of the substances of interest (e.g., bioavailability, persistence in the environment, interaction with other substances)? Highlight any factors identified during your exposure or health effects evaluations that might make a particular exposure more or less likely. For example, explain why the presence of a particular metal in soil is not expected to be bioavailable and therefore unlikely to cause harm. On the other hand, explain why long-term exposures to detected levels of PCBs in fish, for example, might, under site-specific conditions, warrant more concern.
- Are there any populations that might be at increased risk? At a minimum, your document should have a stand-alone section describing child health issues. Carefully examine demographic information for particular groups on or near the site who, based on your review of substance-specific information, might be especially sensitive to toxic effects. Any suspected high-risk groups should be specifically identified in the public health assessment report. Where possible quantify the number and proximity of people in high-risk populations, recognizing that such information might not be readily available.
- What conclusions can be drawn looking at the overall site- and substance-specific information? Depending upon site-specific exposure conditions, you and your team will have a variety of information to sort through and pull together. Ultimately, you will need to make a qualitative judgment about the direction in which the available information leads you. That is, how strong is the evidence suggesting the potential for harm compared to that suggesting no potential for harm? As mentioned earlier, finding a definite answer to whether a harmful effect will occur is generally not possible. Again, your job—to the extent possible—is to put the exposures in perspective. This will enable you and the site team to identify those exposures, if any, that warrant further public health action (see Chapter 9).
- How do missing information or uncertainties limit the conclusions that can be drawn? The strengths, weaknesses, and uncertainties of contributing evidence should be highlighted and organized to support your public health conclusions. Clearly state those instances when, because of weak or missing exposure or health effects data, an answer is not possible.
The two hypothetical scenarios presented below help illustrate the concepts presented in this chapter, including sample narratives. The first example offers a somewhat exaggerated set of conditions to emphasize the components of an in-depth analysis and the decision-making process. The second example presents a scenario in which exposure doses fall closer to observed effect levels, but in the past only; it illustrates the way in which observations and uncertainties would be communicated.
|
EXAMPLE #1: Chemical X (incidental soil ingestion) Site-specific dose = 0.0005 mg/kg/day Health effects data
Exposure data (see Chapters 5 , 6, and 7)
Suggested narrative ATSDR concludes that site exposures to detected levels of Chemical X are not expected to result in harmful health effects. Even assuming that trespassers frequent the site and are exposed to the highest detected concentrations of Chemical X, estimated exposure doses are more than 200 times lower than doses reported to show no harmful effects in experimental (animal) studies and 2,000—20,000 times lower than the lowest doses shown to cause mild health effects in various studies (ATSDR 2000). Also, because Chemical X is more "bioavailable" (that is, more likely to be absorbed and distributed in the body) in water than in a soil matrix, using drinking water studies to evaluate soil exposures may not be entirely appropriate (Smith et al. 1999). However, what it suggests is that even higher exposure doses of Chemical X from site soils would likely be needed to produce the same effects reported in the drinking water studies. The likelihood of exposure to Chemical X from soils is further reduced by the presence of grassy cover throughout the area of concern. Little human data are available to provide added perspective, but two studies of people ingesting Chemical X in their drinking water reported no adverse health effects at doses 40,000 times higher than our site doses (Stillwater 1998). Lastly, scientific studies have shown that Chemical X might behave differently in rodent species than in humans. Studies show that the primary breakdown product (metabolite) of Chemical X thought to be responsible for its toxic effects is not produced in humans (ATSDR 2000). All of these factors taken together strongly suggest that detected levels of Chemical X pose no hazard to area residents. |
|
EXAMPLE #2: Chemical Y (groundwater ingestion) Site-specific dose - 0.02 mg/kg/day Health effects data
Exposure data (see Chapters 5 , 6, and 7)
Suggested narrative After a review of available exposure and health effects data, ATSDR concludes that exposure to Chemical Y in a single well downgradient of the site poses a past hazard. Estimated exposure doses fall below observed effect levels in human studies, but by only less than 10 times. No documentation exists regarding completely "safe" levels (i.e., no NOAEL has been reported at doses below 0.15 mg/kg/day). Given the narrow range between estimated doses and observed effects in humans and the uncertainties about lower dose exposures, a hazard cannot be ruled out. We know that Chemical Y is classified by EPA as a possible human carcinogen, but evidence of cancer effects have been reported in animals only and at doses at least 10,000 times our site-specific doses. Cancer effects are therefore not of concern. Because the affected well was decommissioned and all residences in areas downgradient of the site are served by public water, no current or future hazard exists. Further, site cleanup has reduced the concentration of Chemical Y in the groundwater beneath the site. Detected contaminant concentrations, including Chemical Y, in all other wells in the site vicinity are thousands of times lower than those believed to cause adverse health effects. Therefore, these wells present no past, current, or potential future hazard. |
Alexander M. 2000. Aging, bioavailability, and overestimation of risk from environmental pollutants. Environ Sci Technol 34(20).
ATSDR. 1993. Cancer policy framework. Atlanta: US Department of Health and Human Services. http://www.atsdr.cdc.gov/cancer.html. January 1993.
ATSDR. 1998. Promoting Children's Health, Progress Report of the Child Health Workgroup, Board of Scientific Counselors, 1998-1999.
ATSDR 1999a. Toxicological profile for uranium. Atlanta: US Department of Health and Human Services. September 1999.
ATSDR. 1999b. Child health: guidance for the preparation of toxicological profiles. Atlanta: US Department of Health and Human Services. July 1999.
ATSDR. 2000. Toxicological profile for arsenic. Atlanta: US Department of Health and Human Services. September 2000.
ATSDR. 2001a. Guidance manual for the assessment of joint toxic action of chemical mixtures. Atlanta: US Department of Health and Human Services. Draft for Public Comment. February 2001.
ATSDR. 2001b. Summary report. Hair analysis panel discussion: exploring the state of the science. Prepared by Eastern Research Group, Inc. for ATSDR. Atlanta: US Department of Health and Human Services. http://www.atsdr.cdc.gov/HAC/hair_analysis/. December 2001.
ATSDR. 2002. Case studies in environmental medicine. Pediatric environmental health. US Department of Health and Human Services. July 2002.
Bogdanffy MS, Daston G, Faustman EM, Kimmel CA, Kimmel GL, Seed J, and Vu V. 2001 Harmonization of cancer and noncancer risk assessment: proceedings of a consensus-building workshop. Toxicological Sciences 61:18-31.
Calabrese EJ. 1986. Age and susceptibility to toxic substances. New York: J.H. Wiley and Sons.
Calabrese EJ. 1994. High-risk population groups. Protecting those with genetic predisposition to adverse effects following exposure to chemicals. Occupational Medicine. Third edition. C. Zenz, OB Dickerson, and EP Horvath, Jr., editors. Mosby-Year Book, Inc. St. Louis, Misssouri. pp 800-12.
CDC. National Health and Nutrition Examination Survey (NHANES). Centers for Disease Control and Prevention. MACROBUTTON HtmlResAnchor http://www.cdc.gov/nchs/nhanes.htm
Centers for Disease Control and Prevention. 2003. Second National Report on Human Exposure to Environmental Chemicals.
Crump KS. 1984. A new method for determining allowable daily intakes. Fundam. Appl. Toxicol. 4:854-71.
EPA. 2001. Toxicological review of chloroform (CAS 67-66-3). In support of summary information on the Integrated Risk Information System (IRIS). EPA/635/R-01/001. October 2001.
EPA. 2003a. Draft final guidelines for carcinogen risk assessment final (external review draft). U.S. Environmental Protection Agency, Risk Assessment Forum, Washington, DC, NCEA-F-0644A. March 2003.
EPA. 2003b. Supplemental guidance for assessing cancer susceptibility from early-life exposure to carcinogens (external review draft). U.S. Environmental Protection Agency, Risk Assessment Forum Technical Panel, Washington, DC, EPA/630/R-03/003. February 2003.
Feron VJ, Jonker D, Groten JP, Horbach GJ, Cassee FR, Schoen ED, Opdam JJG. 1993. Combination technology: from challenge to reality. Toxicology Tribune 14:1-3.
FDA. 1993. Guidance document for arsenic in shellfish. Washington: US Department of Health and Human Services, Public Health Service, Food and Drug Administration, Center for Food Safety and Applied Nutrition. January 1993.
Francesconi KA, Edmonds JS. 1997. Arsenic and marine organisms. Advances in Inorganic Chemistry 44:147-89.
Gough M. Antagonism—no synergism—in pairwise tests of carcinogens in rats. Regulatory Toxicology and Pharmacology 35:383-92.
Groten JP. 2000. Mixtures and interactions. Food Chem Toxicol 38(1 Suppl):S65
Hall EJ. 2000. Radiobiology for the radiologist. Philadelphia: Lippincott Williams and Wilkins.
Hardman, JG, Gilman, AG, Limbird, LE, editors. 1995. Goodman and Gilman's Pharmacological Basis of Therapeutics (9th edition). McGraw-Hill, New York. Chapter 1.
HarkinsDK and Susten AS. 2003. Hair analysis: exploring the state of the science. Env Health Perspect 111(4):576-578.
Hill AB. 1965. The environment and disease: association or causation? Proc R Soc Med 58:295-300.
Jonker D, Woutersen RA, van Bladeren PJ, Til HP, and Feron VJ. 1990. 4-week toxicity study of a combination of eight chemicals in rats: Comparison with the toxicity of the individual compounds. Food Chem Toxicol 28(9): 623-631.
Jonker D, Woutersen RA, van Bladeren PJ, Til HP, and Feron VJ. 1993. Subacute (4-week) oral toxicity of a combination of four nephrotoxins in rats: Comparison with the toxicity of the individual compounds. Food Chem Toxicol 31(2): 125-136.
Jorgenson TA, Meierhenry EF, Rushbrook CJ et al. 1985. Carcinogenicity of chloroform in drinking water to male Osborne-Medel rats and female B6C3F1 mice. Fundam Appl Toxicol 5:760-69.
Kimmel CA and Gaylor DW. 1988. Issues in qualitative and quantitative risk analysis for developmental toxicology. Risk Anal 8(1):15-20.
Krishnan K, Haddad S, Beliveau M, and Tardif R. 2002. Physiological modeling and extrapolation of pharmacokinetic interactions from binary to more complex chemical mixtures. Environ Health Perspect 110 (Suppl 6):989-94.
Larson JL, Wolf DC, Butterworth, BE. 1994. Induced cytotoxicity and cell proliferation in the hepatocarcinogenicty of chloroform in female B6C3F1 mice. Comparison of administration by gavage in corn oil vs. ad libitum in drinking water. Fundam Appl Toxicol 23:537-43.
Larson JL, Templin MV, Wolf DC, Jamison KC, Leininger JR et al. 1996. A 90-day chloroform inhalation study in female and male B6C3F1 mice: implications for cancer risk assessment. Fundam Appl Toxocil 30(1):118-37.
Mahaffey KR and Fowler BA. 1977. Effects of concurrent administration of lead, cadmium, and arsenic in the rat. Environ Health Perspect 19: 165-71.
Rios R, Poje GV, Detels R. Susceptibility to environmental pollutants among minorities. Toxicology and Industrial Health 9(5):797-820.
Rothman K. 1986. Modern epidemiology. Boston: Little, Brown and Co.
Seed J, Brown R, Olin SS, Foran JA. 1995. Chemical mixtures: current risk assessment methodologies and future directions. Regul Toxicol Pharmacol 22:76-94.
Stacey NH. 1989. Toxicity of mixtures of trichloroethylene, tetrachloroethylene, and 1,1,1-trichloroethane: Similarity of in vitro and in vivo responses. Toxicol Ind Health 5(3) 441-450.
Susser M. 1973. Chapter 11: criteria for judgment. In: Susser M. 1973. Causal thinking in the health sciences—concepts and strategies of epidemiology. New York: Oxford University Press.
Wade MG, Foster WG, Younglai EV, McMahon A, Leingartner K et al. 2002. Effects of subchronic exposure to a complex mixture of persistent contaminants in male rats: systemic, immune and reproductive effects. Toxicol Sci 67:131-43.
Van Sassenbroeck DK, De Paepe P, Belpaire FM, and Buylaert WA. 2003. Characterization of the pharmacokinetic and pharmacodynamic interaction between gamma-hydroxybutyrate and ethanol in the rat. Toxicol Sci 73:270-8.
ATSDR Science Page, presented by the Office of the Associate Administrator for Science (http://www.atsdr.cdc.gov/cx.html), with links to government and non-government scientific resources.
National Library of Medicine (http://www.nlm.nih.gov/databases): PubMed and Toxnet
EPA Integrated Risk Information System (IRIS) (http://www.epa.gov/iris/), including peer-reviewed toxicologic reviews for selected substances.
EPA Region III Risk Information page, including Region III risk-based concentrations (http://www.epa.gov/reg3hwmd/risk/riskmenu/htm).
International Agency for Research on Cancer (IARC) Monographs (http://monographs.iarc.fr)
National Toxicology Program (NTP) (http://ntp-server.niehs.nih.gov/)
Toxicology Excellence for Risk Assessment (TERA) (http://www.tera.org/)
Ottoboni MA, editor. 1991. The dose makes the poison: a plain language guide to toxicology. 2nd ed. New York: Nostrand Reinhold.
ATSDR. 1997. Healthy children—toxic environments. Report of the Child Health Workgroup. Atlanta: US Department of Health and Human Services.
Bearer C. 1995. How are children different from adults? Environ Health Perspect 103(6):7-12.
EPA. Children's Environmental Health and Safety Inventory of Research (CHEHSIR).
El-Masri HA, Reardon KF, Yang RSH. 1997. Integrated approaches for the analysis of toxicologic interactions of chemical mixtures. Crit Rev Toxicol 27(2):175-97.
EPA. 1986. Guidelines for health risk assessment of chemical mixtures. 51 FR 34014. September 24, 1986.
Groten JP, Feron VJ, Suhnel J. 2001. Toxicology of simple and complex mixtures. Trends Pharmacol Sci 22(6):316-22.
Mumtaz MM, DeRosa CT, Groten J, Feron VJ, Hansen H, Durkin PR. 1998. Estimation of toxicity of chemical mixtures through modeling of chemical interactions. Environ Health Perspect 106(Suppl 6):1253-60.
Mumtaz MM, Next RE, Lichtveld MY, Lewis CR. 1994. The public health impact of chemicals and chemical mixture by-products at hazardous waste sites. In: Andrew JS et al., editors. Hazardous waste and public health: International Congress on the Health Effects of Hazardous Waste. Princeton: Princeton Scientific Publishing Co. pp. 508-16.
|
Toxicology Terminology Adverse effect. A change in physiologic function or cellular structure that is detrimental to the organism. An abnormal or harmful effect. Biologically effective dose. The amount of the absorbed dose reaching the cells or target sites where adverse effect occurs and needed to produce a biologic response. The potential for an observed adverse effect once a biologic response is elicited is dependent on a host of factors including the type of action, repair mechanisms, metabolism, etc. Cancer effects level. The lowest dose level observed to produce a significant increase in the incidence of cancer or tumors (as shown in human epidemiologic or experimental animal studies). Effect. Any change in physiologic function or cellular structure. Exposure. The amount of substance or radiation present in the environment that represents a potential to cause harm to living organisms. Exposure dose. The mathematical estimation of the amount of a substance encountered in the environment per unit of body weight and time. Internal or absorbed dose. The amount of the exposure dose that actually enters the body (i.e., penetrates barriers such as the skin, gastrointestinal tract, lung tissue). The route of exposure, type and form of a substance, among other factors influence how much of a substance is absorbed into the bloodstream. LOAEL (Lowest Observed Adverse Effect Level). The lowest dose level at which an adverse or toxic effect has been observed (from human epidemiologic or experimental animal studies). Mechanism of action. The specific cellular or molecular events (changes, interactions, and alterations) that lead to a specific adverse effect. Mode of action. The overall means by which a chemical produces its adverse effect (e.g., enzyme inhibition, DNA adduct formation). A more general term than mechanism of action (see above). NOAEL (No Observed Adverse Effect Level). Highest dose level (below the LOAEL) at which no adverse or toxic effect has been observed (from human epidemiologic or experimental animal studies-from an individual study). Non-threshold. Based on the theory that a single molecular event can trigger an adverse outcome. Many carcinogens are assumed to function under this principle. The response is considered to be linear throughout the dose range. The theory leads to a mathematical model that generates a single number, the cancer slope factor (CSF) which relates risk to dose, regardless of the size of the dose (the CSF is used in quantitative risk assessments). Pharmacodynamics. The study of biochemical and physiological effects of substances and their mechanisms of action. Threshold. The lowest dose of a substance at which a measurable adverse effect is observed. Toxicokinetics (Pharmacokinetics).The study of the kinetics (movement) of toxic substances within the body. Specifically, the study of the absorption, distribution, metabolism (biotransformation), and excretion of a substance. Toxicology. The study of the adverse effects that chemicals may have on living organisms. It involves understanding how a substance gets into the body, how it is able to exert what adverse effects, how to prevent or mitigate those effects, and the amount of substance required to result in each adverse effect (i.e., the dose-response relationship). |
2 The evaluation of non-cancer and cancer endpoints are described separately in this manual because of the non-cancer/cancer dichotomy used historically in quantitative risk assessment and in the derivation of many health- based screening values. (See the text box in Section 8.3.1 for a brief discussion on the movement toward harmonizing the approaches used to evaluate non-cancer and cancer endpoints and its relevance to the in-depth analysis described in this chapter.)
3 In some cases, the health guideline can be based on benchmark dose" (BMD) or "point of departure." The benchmark dose method involves fitting mathematical models to the available dose-response data (from single or multiple studies) and using the results to select a dose associated with a specified low level of risk (e.g., a 5% or 10% increase in the incidence of stomach lesions). Scientists have long recognized the limitations of relying on the NOAEL from a single study when deriving health guideline values (Crump 1984; Kimmel and Gaylor 1988). The approach is limited to a single dose within a study and is dependent on study design. It does not account for the variability in the estimate of the dose-response and does not account for the slope of the dose-response curve.
4 See Appendix F for further discussion on how EPA derives its cancer slope factor and its current approach to cancer risk assessment.
- Page last reviewed: November 5, 2015
- Page last updated: November 5, 2015
- Content source:



 ShareCompartir
ShareCompartir