Appendix F: Derivation of Comparison Values
ATSDR has developed health guidelines and environmental guidelines to use when conducting the screening analysis and evaluating exposures to substances found at sites under investigation. Health guidelines are substance-specific doses or concentrations derived using toxicologic information. Where adequate dose-response data exist, health guidelines are derived for both the ingestion or inhalation routes of exposure. Health guidelines include ATSDR's minimal risk levels (MRLs). No health guidelines have been developed by ATSDR for dermal exposures. Environmental guidelines are media-specific substance concentrations derived from health guidelines using default exposure assumptions. ATSDR environmental guidelines include environmental media evaluation guides (EMEGs), reference dose media evaluation guides (RMEGs), and cancer risk evaluation guides (CREGs) that are available for contact with substances in water, soil, and air. No environmental guidelines have been developed by ATSDR for contact with contaminants in food or biota.
In addition to comparison values derived by ATSDR, other federal and some state agencies have developed similar types of health-based guidelines for concentrations of substances in water, soil, air, and food. You also may use these comparison values, when appropriate, to evaluate exposures to substances detected in various site media.
This appendix provides a description of comparison values available from ATSDR, as well as other sources. Sections 1.0 and 3.0 describe the health and environmental guidelines derived by ATSDR, respectively. ATSDR comparison values include MRLs, EMEGs, RMEGs, and CREGs. These values should receive priority when selecting comparison values. Sections 2.0 and 4.0 describe the health and environmental guidelines derived by other agencies, respectively. These values should be selected as comparison values only when appropriate ATSDR values are not available. Non-ATSDR comparison values discussed in this appendix include: EPA's RfDs, RfCs, CSFs, IURs, RBCs, MCLs, MCLGs, DWELs, HAs, SSLs, NAAQS; FDA's action levels; the National Council on Radiation Protection and Measurements (NCRP) radiation guidelines and (NCRP) soil screening limits; OSHA's PELs; NIOSH's RELs; and ACGIH's TLVs.
For each guideline discussed, a definition and description of the derivation and applicability or intended use are provided to enable you to determine if a comparison value is appropriate to use for evaluating site-specific conditions. For comparison values derived by agencies other than ATSDR, the referenced source(s) is also provided. Because comparison values are frequently revised and updated, any published table of values would soon be outdated. Therefore, numerical values are not presented in this appendix, instead sources in which values can be found are provided.
1.0 ATSDR'S HEALTH GUIDELINES
1.1 Minimal Risk Levels (MRLs)
Definition/Derivation. ATSDR in cooperation with EPA has developed a priority list of hazardous substances found at hazardous waste sites, as directed under the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA), as amended by the Superfund Amendment and Reauthorization Act of 1986 (SARA). For those substances most commonly found, ATSDR has prepared toxicological profiles that include an examination, summary, and interpretation of available toxicologic and epidemiologic data.
Based on the review of the available data, ATSDR has derived MRLs when reliable and sufficient data exist to identify the target organ(s) of effect or the most sensitive health effects(s) for a specific duration for a given route of exposure. MRLs are an estimate of the daily human exposure to a substance that is likely to be without appreciable risk of adverse health effects during a specified duration of exposure. MRLs are based only on noncarcinogenic effects. MRLs are screening values only and are not indicators of health effects. Exposures to substances at doses above MRLs will not necessarily cause adverse health effects and should be further evaluated.
MRLs are set below levels that might cause adverse health effects in most people, including sensitive populations. MRLs are derived for acute (1-14 days), intermediate (15-365 days), and chronic (365 days and longer) durations for the oral and inhalation routes of exposure. Currently, MRLs for dermal exposure are not derived because ATSDR has not yet identified a method suitable for developing MRLs for this route of exposure. MRLs are generally based on the most sensitive chemical-induced endpoint considered to be relevant to humans. Serious health endpoints (e.g., irreparable damage to the liver or kidneys, or birth defects) are not used as a basis for establishing MRLs.
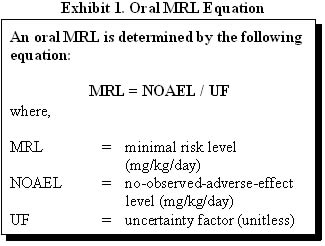
MRLs are derived for substances by factoring the most relevant documented no-observed-adverse-effects level (NOAEL) or lowest-observed-adverse-effects level (LOAEL) and an uncertainty factor. Exhibit 1 demonstrates the derivation of an oral MRL using a NOAEL.
Inhalation MRLs are exposure concentrations expressed in units of parts per billion (ppb) for gases and volatiles, or micrograms per cubic meter (µg/m3) for particles. Inhalation MRLs are derived for continuous, 24-hour a day exposures. The specific approach used to derive MRLs for individual substances are detailed in ATSDR's Toxicological Profile for each substance.
Most MRLs contain a degree of uncertainty because of the lack of precise toxicologic information about the people who might be most sensitive (e.g., children, elderly, those with pre-existing illnesses) to the effects of environmental contamination. ATSDR uses a conservative (i.e., protective) approach to address this uncertainty. This is consistent with the public health principle of prevention. Although human data are preferred, MRLs often must be based on animal studies because relevant human studies are lacking. In the absence of evidence to the contrary, ATSDR assumes that humans are more sensitive to the effects of hazardous substances than animals and that certain persons may be particularly sensitive. Uncertainties are accounted for by applying "uncertainty" factors to the NOAEL. For example, an uncertainty factor of between 1 and 10 may be applied for extrapolation from animal doses to human doses and/or a factor between 1 and 10 may be applied to account for sensitive individuals. When more than one uncertainty factor is applied, the uncertainty factors are multiplied. In this example, the uncertainty factor would be 100—10 for the extrapolation to humans and 10 to account for sensitive individuals.
For example, the MRL for chronic exposures through ingestion of pentachlorophenol is based on a reproductive study of female mink. Mink were exposed to a dose of 1 mg/kg/day from 3 weeks prior to mating until weaning of first-generation offspring. As a result of this exposure, no overt signs of toxicity were observed and no reproductive end points were altered, but serum thryoxine concentrations were reported in first generation males and in males and females in the second generation, along with significantly-decreased relative thyroid weight in females in the second generation. A dose of 1 mg/kg/day was identified as the LOAEL for pentachlorophenol. ATSDR divided the LOAEL by an uncertainty factor of 1,000 when deriving the MRL for pentachlorophenol. The uncertainty factor was based on factors of 10 to extrapolate from a LOAEL to a NOAEL, 10 to extrapolate from animal to human doses, and 10 to account for sensitive individuals, to result in an MRL of 0.001 mg/kg/day. (Note that MRLs are rounded to one significant digit.)
Applicability/Intended Use. MRLs are intended to serve only as a screening tool to help you decide if you should more closely evaluate exposures to a substance found at a site. MRLs are not intended to define cleanup or action levels. Exposure doses above the MRL does not necessarily mean that adverse health effects will occur.
When using MRLs, you should be aware that ATSDR derives MRLs assuming that exposures are occurring to a single substance and that only noncarcinogenic health effects will occur. At hazardous waste sites, people are usually exposed to a mixture of substances. Current scientific evidence indicates that substances can and do interact with each other to alter the substances' toxicities. Interactions may be additive, antagonistic, or synergistic. Because there are an infinite number of possible substance combinations and resulting interactions, only limited information is available to assess these interactions. With the lack of data on interactions, health assessors typically assume toxic effects are additive. You should be aware of the limitations that MRLs have in assessing chemical mixtures and seek information about possible substance interactions. This information can be gathered during the in-depth evaluation described in Chapter 8 of this manual.
MRLs also account only for noncarcinogenic toxic effects of substances. For carcinogenic substances, you follow the steps described in Chapter 8 of this manual, which involves a balanced review and integration of relevant exposure, toxicologic, epidemiologic, and medical data.
2.0 NON-ATSDR HEALTH GUIDELINES
2.1 Subchronic and Chronic Reference Doses (RfDs) and Reference Concentrations (RfCs)
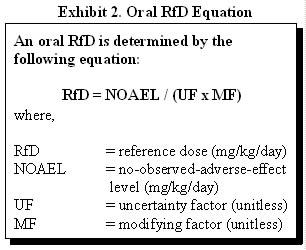
Definition/Derivation. EPA developed chronic RfDs for ingestion and RfCs for inhalation as estimates of daily exposures to a substance that are likely to be without a discernable risk of deleterious effects to the general human population (including sensitive subgroups) during a lifetime of exposure. RfDs and RfCs are doses derived from the NOAEL or LOAEL by application of uncertainty factors and an additional modifying factor, which is based on a professional judgment of the entire database of the chemical. EPA includes uncertainties sometimes spanning orders of magnitude to ensure that the potential for health effects is overestimated. Exhibit 2 demonstrates the derivation of a RfD using a NOAEL.
The subchronic RfD or RfC is an estimate of an exposure level that would not be expected to cause adverse effects when exposure occurs during a limited time interval. Subchronic values are determined from animal studies with exposure durations of 30 to 90 days. Subchronic human exposure information is usually derived from occupational exposures and accidental acute exposures.
Applicability/Intended Use. RfDs and RfCs are based on the assumption that thresholds exist for certain toxic effects such as cell death or organ damage. RfDs and RfCs are derived for the noncarcinogenic health effects of compounds that are also carcinogens. Therefore, it is essential to refer to other sources of information concerning the carcinogenicity of this substance. RfDs and RfCs are also derived assuming exposure to a single substance in a single media. Doses less than the RfD or RfC are not expected to be associated with health risks, but doses less than the RfD or RfC are not necessarily "acceptable," and doses in excess of the RfD or RfC are not necessarily "unacceptable."
References. EPA. 1993. Background Document 1A—Reference Dose (RfD): Description and Use in Health Risk Assessments. http://www.epa.gov/iris/rfd.htm . March 15, 1993.
EPA. 2001. Integrated Risk Information System (IRIS). Office of Research and Development, National Center for Environmental Assessment. http://www.epa.gov/iris/index.html . September 28, 2001.
2.2 Cancer Slope Factor (CSF) and Inhalation Unit Risk (IUR)
Definition/Derivation.
EPA evaluates the potential carcinogenicity of a substance using a two-step process—a qualitative weight-of-evidence approach and a quantitative assessment to define the relationship between dose and the likelihood of a theoretical increase in cancer cases in a population.
Based on the rationale and methods described in EPA's 2003 draft carcinogen risk assessment guidelines, EPA conducts a qualitative weight-of-evidence evaluation of human and animal toxicity studies of a substance. EPA provides weight-of-evidence narratives and presents the following descriptors to describe the carcinogenicity of a given substance:
- Carcinogenic to Humans
- Likely to Be Carcinogenic to Humans
- Suggestive Evidence for Carcinogenic Potential
- Inadequate Evidence to Assess Carcinogenic Potential
- Not Likely to Be Carcinogenic to Humans
Earlier EPA guidelines (1986) used a slightly different cancer classification scheme, which is still in place for many substances. Under that scheme potential carcinogens are classified as follows:
A Human carcinogen (sufficient human data)
B1 Probable human carcinogen (limited human data, sufficient animal data)
B2 Probable human carcinogen (inadequate human data, sufficient animal data)
C Possible human carcinogen (inadequate or no human data, sufficient animal data)
D Not classifiable as to human carcinogenicity (inadequate or no human and animal data)
E Evidence of noncarcinogenicity in humans (adequate human and animal data)
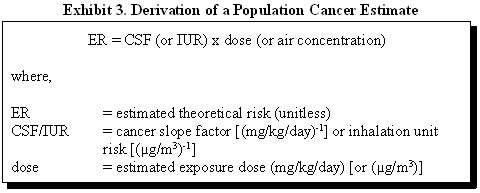
For known or possible carcinogens, CSFs and IURs are used as a quantitative indication of the carcinogenicity of a substance. A CSF is an estimate of possible increases in cancer cases in a population. A CSF is expressed in dose units [(mg/kg/day)-1] to allow for comparison with calculated oral doses, described in Appendix G of this manual. An IUR is an estimate of theoretical increases in cancer cases in a population expressed in concentration units [(µg/m3)-1] to allow for comparison with site-specific air concentrations. Because there can be differences in the carcinogenicity of a substance depending on the route of exposure, a CSF for ingestion exposures or IUR for inhalation exposures should not be applied to a different route of exposure unless there is adequate justification for this assumption.
CSFs and IURs are usually derived from animal experiments that involve exposures to a single substance by a single route of exposure (i.e., ingestion or inhalation). EPA extrapolates CSFs and IURs from experimental data of increased tumor incidences at high doses to estimate theoretical cancer rate increases at low doses. The experimental data often represent exposures to chemicals at concentrations orders of magnitude higher than concentrations found in the environment.
Historically, EPA has used mathematical models, which apply a number of uncertainties and conservative assumptions, to manipulate the experimental data and extrapolate possible health outcomes from high doses to low doses. These mathematical models assume that there are no thresholds for cancer effects (or low dose linearity)—a single molecule of a carcinogen is assumed to be able to cause cancer.
As scientists learn more about how carcinogens produce tumorogenic responses in animals and humans (i.e., the mechanism of action), they are finding that some carcinogens exhibit thresholds. (1) In light of the evolving science, EPA's more recent guidelines call for more emphasis on analyzing the dose-response data before invoking low-dose linear defaults as described above. The new guidelines call for closer examination of substance-specific modes and mechanisms of action. This procedure "weighs" the available evidence, invoking a two-step dose-response process: (1) modeling the observed data to the "point of departure" and (2) extrapolating to lower doses. When data are sufficient, nonlinear extrapolation may be considered. (2) In the absence of adequate data showing nonlinear dose-response, the guidelines call for defaulting to linear assumptions. The concepts described in the guidelines are consistent with ATSDR's approach to assessing carcinogenic substances, as described in Chapter 8 of this manual.
Applicability/Intended Use. EPA assesses the carcinogenicity of a substance both qualitatively and quantitatively. As a result of a qualitative evaluation of information relevant to carcinogenicity and the quality of this information, EPA assigns cancer classifications to suspected carcinogenic substances. The cancer classifications should be discussed when discussing carcinogens in your public health assessment.
EPA develops CSFs and IURs as a result of a quantitative evaluation of a suspected carcinogenic substance. CSFs and IURs are combined with information about exposure doses to estimate a theoretical increase in cancer cases in a population. Risk assessors conducting human health risk assessment using EPA's Risk Assessment Guidance for Superfund (RAGS) (1989), use the following equation to estimate possible excess cancer risks in a population:
Under the quantitative risk assessment method, site-specific cancer doses and concentrations are multiplied by EPA's CSFs or IURs, respectively. This exercise estimates a theoretical excess cancer risk expressed as the proportion of a population that may be affected by a carcinogen during a lifetime of exposure. For example, an estimated cancer risk of 2 x 10-6 represents a possible 2 excess cancer cases in a population of 1 million. Because of the uncertainties and conservatism inherent in deriving the CSFs and IURs, this is only an estimate of risk; the true risk is unknown and could be as low as zero (EPA 2003).
Although ATSDR recognizes the utility of numerical risk estimates in risk analysis, the agency considers such estimates in the context of the variables and assumptions involved in their derivation and in the broader context of biomedical opinion, host factors, and actual exposure conditions. The actual parameters of environmental exposures must be given carefully considered in evaluating the assumptions and variables relating to both toxicity and exposure (ATSDR 1993).
References. ATSDR. 1993. Cancer Policy Framework. U.S. Department of Health and Human Services. January 1993.
EPA. 1989. Risk assessment guidance for Superfund. Volume I. Human health evaluation manual. Interim final. EPA/540/1-89/002. http://www.epa.gov/oerrpage/superfund/programs/risk/ragsa/index.htm
EPA. 2003. Draft final guidelines for carcinogen risk assessment final (external review draft). U.S. Environmental Protection Agency, Risk Assessment Forum, Washington, DC, NCEA-F-0644A. March 2003.
EPA. 2001. What is IRIS? Office of Research and Development, National Center for Environmental Assessment. http://www.epa.gov/iris/intro.htm. August 13, 2001.
3.0 ATSDR'S ENVIRONMENTAL GUIDELINES
3.1 Environmental Media Evaluation Guides (EMEGs)
Definition/Derivation. EMEGs represent concentrations of substances in water, soil, and air to which humans may be exposed during a specified period of time (acute, intermediate or chronic) without experiencing adverse health effects. Acute exposures are defined as those of 14 days or less; intermediate exposures are those lasting 15 days to 1 year; and chronic exposures are those lasting longer than 1 year. EMEGs have been calculated for substances for which ATSDR has developed Toxicological Profiles using information about the substance toxicity (MRLs) and default exposure assumptions. The default exposure assumptions account for variations in water and soil ingestion between adults and children. For exposure to substances in the air, EMEGs are expressed as air concentrations and are the same for adults and children. The derivation of EMEGs is discussed separately under each media section (water, soil, and air), below.
Applicability/Intended Use. EMEGs are used when conducting an environmental guideline comparison during a screening analysis to quickly evaluate large quantities of data for a site under investigation. Substances found at concentrations below EMEGs are not expected to pose public health hazards. Substances found at concentrations above EMEGs require further evaluation before drawing a public health conclusion. When conducting an environmental guideline comparison, you must remember that EMEGs are screening values only, and not indicators of adverse public health effects. Substances found at concentrations above EMEGs will not necessarily cause adverse health effects and should be further evaluated.
In using EMEGs for a environmental guideline comparison, you have several choices of EMEGs based on chronic and intermediate duration exposures for adults and children. For health assessment purposes, you typically assume that chronic exposures to children are possible and use the corresponding EMEG to conduct an environmental guideline comparison. The chronic EMEG for children is usually the lowest EMEG concentration available for a substance and represents the most conservative, or protective, assumptions when conducting a screening analysis. When chronic exposures or child exposures can be excluded, the intermediate EMEGs or adult EMEGs may be the most appropriate values for conducting screening. You can also derive an EMEG from an acute MRL, as described later in this section of the appendix, when only short-term exposures are occurring. You are encouraged to consider all available site-specific conditions about the possible exposure durations and possibly exposed populations when selecting the most appropriate EMEG.
You should, however, recognize the limitations of EMEGs. ATSDR makes three assumptions when deriving EMEGs: 1) exposures are occurring through contact to a single medium, 2) exposures are occurring to a single substance, and 3) only noncarcinogenic health effects will occur.
Although EMEGs assume exposures are occurring through contact with a substance in a single medium, a person could be concurrently exposed to the substance in multiple media (e.g., water, soil, air, or food). The relative contribution of a particular exposure pathway to the total amount of a substance that a person contacts can vary dramatically depending on site-specific circumstances. Because of site-to-site variability, it is infeasible for ATSDR to develop EMEGs that account for possible exposures from multiple pathways. Therefore, if exposure to a substance is occurring by multiple exposure pathways, you should consider conducting a health guideline comparison during the screening process, as described in Chapter 7 and Appendix G of this guidance manual.
EMEGs are derived assuming that exposures are occurring from a single substance. More often than not, hazardous waste sites contain a mixture of substances to which people will be exposed. A growing body of scientific information exists documenting the occurrence of interactive effects from simultaneous exposures to two or more substances. Such interactions may be additive, antagonistic, or synergistic. Most studies that have documented interactions have resulted from exposures where mixture components are in the observable effects range, not at concentrations at or below NOAELs—the dose levels from which EMEGs are derived. Studies that have examined exposures to lower concentrations suggest that exposure to a mixture of chemicals is unlikely to produce adverse health effects as long as components of that mixture are detected at levels well below NOAEL for individual contaminants. While no set of environmental guidelines could account for the infinite array of substances in varying proportions that may be found at sites, it is reasonable to conclude that if detected levels of chemicals are individually below health-based screening values described in this appendix, then exposure to these chemicals collectively is not expected to be of health concern.
EMEGs are based on toxicity information (MRLs), which consider noncarcinogenic toxic effects of chemicals, including their developmental and reproductive toxicity. MRLs do not consider potential genotoxic or carcinogenic effects of a substance. Because some substances have both noncarcinogenic and carcinogenic effects, ATSDR has derived CREGs to consider potential carcinogenic effects of a substance. CREGs are discussed in more detail in Section 3.3 of this appendix.
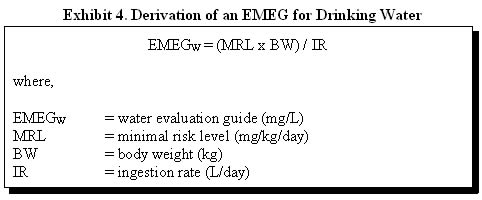
Water EMEGs. Water EMEGs are derived for potable water used in homes. Potable water includes water used for drinking, cooking, and food preparation. Exposures to substances that volatilize from potable water and are inhaled, such as volatile organic compounds (VOCs) released during showering, are not considered when deriving EMEGs. More information about exposures to substances from volatilization is discussed in Appendix G . For potable water exposures, an EMEG is derived from the following equation:
To derive the water EMEGs, ATSDR uses the chronic oral MRLs from the Toxicological Profiles. Ideally, the MRL is based on an experiment in which the chemical was administered in water. However, in the absence of such data, an MRL based on an experiment in which the chemical was administered by gavage or in food may have been used. The Toxicological Profiles for individual substances provide detailed information about the MRL and the experiment on which it was based.
Children are usually assumed to constitute the most sensitive segment of the population for water ingestion because their ingestion rate per unit of body weight is greater than the adults' rate. An EMEG for a child is calculated assuming a daily water ingestion rate of 1 liter per day (L/day) for a 10-kilogram (kg) child. For adults, a water EMEG is calculated assuming a daily water ingestion rate of 2 liters per day and a body weight of 70 kg. According to the U.S. Environmental Protection Agency's (EPA's) Exposure Factor's Handbook (EPA 1997), the average adult and child (ages 1 through 10 years) water intake rates are 1.4 L/day and 0.74 L/day, respectively. The 90th percentile drinking water intake rates for an adult and child are 2.3 L/day and 1.3 L/day, respectively. The body weights are based on an average infant (6 to 11 months) body weight of 9.1 kg and an average adult body weight of 71.8 kg (EPA 1997). Concentrations of substances in water are expressed as milligrams per liter (mg/L) or parts per million (ppm).
For example, ATSDR derived the EMEG for a child and adult exposed to 1,1-dichloroethene in drinking water as follows.
Reference Child (chronic exposures)
EMEGW = (0.009 milligrams per kilogram per day [mg/kg/day] (3) x 10 kg) / (1 L/day)
EMEGW = 0.09 mg/L (4)
EMEGW = (0.009 mg/kg/day1 x 70 kg) / (2 L/day)
EMEGW = 0.3 mg/L
To derive the soil EMEGs, ATSDR uses the chronic oral MRLs from its Toxicological Profiles. Many chemicals bind tightly to organic matter or silicates in the soil. Therefore, the bioavailability of a chemical is dependent on the media in which it is administered. Ideally, an MRL for deriving a soil EMEG should be based on an experiment in which the chemical was administered in soil. However, data from this type of study is seldom available. Therefore, often ATSDR derives soil EMEGs from MRLs based on studies in which the chemical was administered in drinking water, food, or by gavage using oil or water as the vehicle. The Toxicological Profiles for individual substances provide detailed information about the MRL and the experiment on which it was based.
Children are usually assumed to be the most highly exposed segment of the population because their soil ingestion rate is greater than adults' rate. Experimental studies have reported soil ingestion rates for children ranging from approximately 40 to 270 milligrams per day (mg/day), with 100 mg/day representing the best estimate of the average intake rate (EPA 1997). ATSDR calculates an EMEG for a child using a daily soil ingestion rate of 200 mg/day for a 10-kg child.
For sites where the only receptors for soil ingestion are adults, an EMEG is calculated using an adult body weight of 70 kilograms and an assumed daily soil ingestion rate of 100 mg/day. There are very few data on soil ingestion by adults, but limited experimental studies suggest a soil ingestion rate in adults of up to 100 mg/day, with an average intake of 50 mg/kg (EPA 1997). Concentrations of substances in soil are expressed as milligrams per kilogram (mg/kg) or ppm.
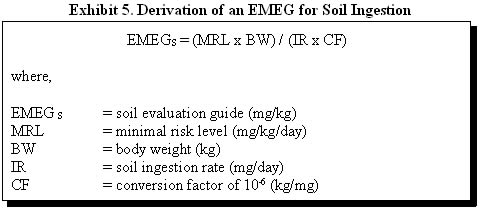
For example, ATSDR derived the EMEG for a child and adult exposed to 1,1-dichloroethene in soil as follows:
Reference Child (chronic exposure)
EMEGS = (0.009 mg/kg/day x 10 kg) / (200 mg/day x 10-6 kg/mg)
EMEGS = 500 mg/kg
EMEGS = (0.009 mg/kg/day x 70 kg) / (100 mg/day x 10-6 kg/mg)
EMEGS = 6000 mg/kg
ATSDR also develops EMEGs for soil-pica exposures. Soil-pica involves ingestion of soils at unusually high rates that greatly exceed most of the population (1,000-5,000 mg/day) (ATSDR 2001). The distribution of soil-pica ingestion rates has not been well-characterized. Most exposure data related to soil-pica behavior is based on observations of only a few children conducted during a short period (2 weeks or shorter), not accounting for frequency of or variations in this behavior. These studies report daily ingestion rates ranging up to 50,000 mg, with a 95th percentile soil ingestion rate reported at 208 mg/day (ATSDR 2001; Calabrese and Stanek, 1998; EPA 1997). Based on available data, ATSDR uses a soil ingestion rate of 5,000 mg/day for a 10-kg child in developing child pica EMEGs. This is considered a conservative default value (ATSDR 2001). ATSDR does not develop child pica EMEGs for chronic exposures because exposures are expected to be more intermittent (i.e., of an acute or intermediate nature).
Air EMEG. EMEGs for inhalation exposures to airborne contaminants are derived from the chronic inhalation MRLs presented in the ATSDR Toxicological Profiles or ATSDR's HazDat database. The inhalation MRLs are expressed in concentration units of micrograms/cubic meter (µg/m3) or parts per billion (ppb). Therefore, the air EMEG for a chemical is the same as its MRL, and no mathematical calculation is required. The same air EMEG value is used for all segments of the population. For chemical substances that exist in a vapor form at standard temperature and pressure (STP), the value is given in ppb (volume basis); for substances that are solids at STP, the value is given in µg/m3.
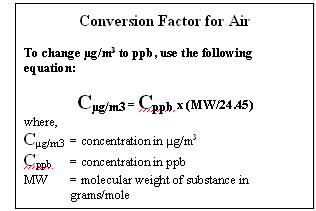
ATSDR MRLs are derived for continuous, 24-hour a day exposures. In many instances, inhalation exposures from a site may be for less than 24 hours per day. Therefore, the use of air EMEGs based on MRLs to assess these situations would provide a conservative approach for identifying air contaminants of potential health concern.
For some chemicals, there may be experimental toxicity data in which the chemical was administered orally, but no data in which the chemical was administered by inhalation. Significant differences may exist in the toxicity of the chemical for oral ingestion as compared to inhalation exposure because of differences in the absorption, metabolism, distribution, and site-specific toxicity of the chemical. Therefore, an air EMEG is derived only from a MRL that is based on an inhalation study.
3.2 Reference Dose Media Evaluation Guides (RMEG)
Definition/Derivation. If no MRL is available to derive an EMEG, ATSDR develops RMEGs using EPA's reference doses (RfDs) and default exposure assumptions, which account for variations in intake rates between adults and children. EPA's reference concentrations (RfCs) serve as RMEGs for air exposures. Like EMEGs, RMEGs represent concentrations of substances (in water, soil, and air) to which humans may be exposed without experiencing adverse health effects. RfDs and RfCs consider lifetime exposures, therefore, RMEGs apply to chronic exposures.
Like EMEGs, RMEGs are developed assuming: 1) exposures are occurring through contact to a single medium, 2) exposures are occurring to a single substance, and 3) only non-carcinogenic health effects will occur. As such, you should be aware of the limitations associated with using RMEGs, which are the same as the limitations of using EMEGs described in Section 1.1 of this appendix.
Applicability/Intended Use. When no EMEGs are available, RMEGs serve as a screening tool to be used when conducting an environmental guideline comparison. Like EMEGs, substances found at concentrations below RMEGs are not expected to pose public health hazards and substances found at concentrations above RMEGs require further evaluation before drawing a public health conclusion. RMEGs also serve only as screening values and not indicators of public health hazards.
In selecting the RMEG that represents the possibly exposed population, you typically assume that exposures to children are possible. The RMEG derived for childhood exposures, therefore, should be used for assessing substance concentrations unless childhood exposures can be excluded. Because RMEGs are derived assuming chronic exposures, they should be used only for long-term (greater than a year) exposures.
3.3 Cancer Risk Evaluation Guides (CREGs)
Definition/Derivation. CREGs are media-specific comparison values that are used to identify concentrations of cancer-causing substances that are unlikely to result in an increase of cancer rates in an exposed population. ATSDR develops CREGs using EPA's cancer slope factor (CSF) or inhalation unit risk (IUR), a target risk level (10-6), and default exposure assumptions. The target risk level of 10-6 represents a theoretical risk of 1 excess cancer cases in a population of 1 million (5). The default exposure assumptions account for ingestion rates and body weights. CREGs are only available for adult exposures—no CREGs specific to childhood exposures are available.
In developing the CREGs, ATSDR assumes that 1) exposures occur through contact to a single medium, 2) exposures occur to a single substance, and 3) only cancer health effects will occur. As such, you should be aware of the limitations associated with using CREGs, which are similar to the limitations of using EMEGs described in Section 3.1 of this appendix. More information about the derivation of CREGs are included in the discussion of each media (water, soil, and air), below.
Applicability/Intended Use. CREGs serve as a screening tool for evaluating concentrations of carcinogens during an environmental guideline comparison. CREGs should be used only when assessing exposures to adults; CREGs for children have not yet been developed. You should also remember that CREGs are based on theoretical estimates of cancer risk. CREGs should, therefore, serve only as a screening tool and not as an indication that cancer is expected or predicted.
Water and Soil CREGs. Like EMEGs, water CREGs are derived for potable water used in homes, including water used for drinking, cooking, and food preparation. Soil CREGs apply only to soil that is ingested. Water and soil CREGs are derived from the following equation:

In understanding this equation, remember that a theoretical risk is calculated by multiplying the dose and the CSF, as described in Appendix G . When developing the CREG, the target risk level (10-6), which represents a theoretical risk of 1 excess cancer case in a population of 1 million, and the CSF are known. The calculation seeks to find the substance concentration and dose associated with this target risk level.
To derive the water and soil CREGs, ATSDR uses CSFs developed by EPA and reported in the Integrated Risk Information System (IRIS). The IRIS summaries, available at http://www.epa.gov/iris/ , provide detailed information about the derivation and basis of the CSFs for individual substances. ATSDR derives CREGs for lifetime exposures, and therefore uses exposure parameters that represent exposures as an adult. An adult is assumed to ingest 2 L/day of water and weigh 70 kg. For soil ingestion, ATSDR assumes a soil ingestion rate of 100 mg/day.
For example, ATSDR derived CREGs for lifetime exposures to vinyl chloride through ingestion of drinking water or soil as follows:
Lifetime Drinking Water Exposure
CREGW = (10-6 x 70 kg) / (2 L/day x 1.4 (mg/kg/day)-1 )
CREGW = 0.00003 mg/L
CREGS = (10-6 x 70 kg) / (100 mg/day x 10-6 kg/mg x 1.4 (mg/kg/day)-1 )
CREGS = 0.5 mg/kg
To derive the air CREGs, ATSDR uses IURs developed by EPA and reported in IRIS. Because toxicity studies of inhalation exposures express doses as concentrations, the IURs are estimates of the theoretical risk of cancer associated with a carcinogen expressed in concentration units. As such, no exposure parameters for intake rate or body weight are needed to derive CREGs for inhalation exposure. ATSDR assumes, however, that exposure is continuous—occurring for 24 hours a day.
For example, the CREG for lifetime exposures to vinyl chloride through inhalation is as follows:
Lifetime Inhalation Exposures
CREGA = 10-6 / 0.000009 g/m3
CREGA = 0.1 g/m3
4.0 NON-ATSDR ENVIRONMENTAL GUIDELINES
When ATSDR values are not available, environmental guideline from other sources, such as those described below can be considered. Before using non-ATSDR derived guidelines, however, it is important to understand the derivation and underlying use of that guideline to ensure that screening a substance against it is appropriate. Generally, only human health-based values should be considered.
4.1 EPA Region 3 Risk-Based Concentrations (RBCs)
Definition/Derivation. EPA Region 3 Risk Based Concentrations (RBCs) are guidelines used to assess the potential for harm from chemicals found at a hazardous waste site. They are developed by combining a substance's toxicologic properties with "standard" scenarios for encountering the substance. EPA's measures of a substance's toxicologic properties are the RfD and CSF. The RfD is the dose of a chemical not expected to result in noncarcinogenic health effects, and the CSF is the cancer risk per unit dose. Exposure scenarios are taken from RAGS or Superfund supplemental guidance. The exposure parameters are generic and are intended to be overly conservative and protective of most populations. EPA uses these standard exposures to determine the exposure dose equivalent of the RfD or target cancer risk level. EPA Region 3 has compiled RBCs for 400 to 500 substances in soil, air, water, and fish. RBCs are presented by EPA Region 3 in the RBC Table, which is generally updated every 6 months.
Applicability/Intended Use. EPA Region 3 developed the RBC Table as a tool to aid Superfund risk assessors in screening substances at hazardous waste sites. RBCs are also used for responding to citizen inquiries and spot-checking baseline risk assessments.
RBCs have some important limitations. Each RBC is estimated assuming a person is exposed to a single substance in a single media. They do not consider the transfer of substances from soil to air or dermal contact with a substance. Toxicity information in the RBC Table was calculated by hand, and though the Table has been checked several times, it may contain errors. Therefore, EPA Region 3 emphasizes that RBCs are not intended to be used as regulatory cleanup goals. RBCs do not consider site-specific exposure scenarios because they are derived from generic exposure parameters. However, they can be used as an initial screening of substances found in site media.
References. EPA. 1989. Risk Assessment Guidance for Superfund, Volume I: Human Health Evaluation Manual (Part A). Office of Solid Waste and Emergency Response, Toxics Integration Branch. Publication No.: EPA/540/1-89/002. December 1989.
EPA Region 3 Hazardous Site Cleanup Division. Risk Assessment. EPA Region III Risk-Based Concentration Table. http://www.epa.gov/reg3hwmd/risk/riskmenu.htm.
4.2 EPA Maximum Contaminant Levels (MCLs)
Definition/Derivation. The Safe Drinking Water Act (SDWA) establishes national primary drinking water regulations in the form of maximum contaminant levels (MCLs). MCLs are enforceable drinking water regulations that are protective of public health, but also consider economic and technological constraints. Consideration of economic and technological constraints does not imply that MCLs are set above levels harmful to human health. Rather, MCLs represent more realistic assumptions about toxicity and contain fewer uncertainty factors than the very conservative ATSDR environmental guidelines. National primary drinking water regulations apply to all public water systems including community water systems and transient and nontransient noncommunity water systems.
An MCL is the maximum permissible level of a contaminant in water that is delivered to the free-flowing outlet of the ultimate user of a public water system. Contaminants added to the water by the user, except those resulting from corrosion of piping and plumbing caused by water quality, are exempt from meeting MCLs. In setting MCLs, EPA considers health implications from possible exposures, as well as available technology, treatment techniques, and other means to reduce contaminant concentrations. Cost of implementing technologies is also considered.
MCLs are deemed protective of public health during a lifetime (70 years) at an exposure rate of 2 L/day. MCLs are dynamic values, subject to change as water treatment technologies and economics evolve and/or as new toxicologic information becomes available.
Applicability/Intended Use. MCLs are the heart of the national primary drinking water regulations, and have been issued by EPA under the authority of the SDWA. Drinking water standards in the United States were originally promulgated in 1914; they were reissued or revised in 1925, 1942, 1946, and 1962. While the 1914 drinking water standards were concerned solely with bacteriologic quality, the 1925 standards and those of following years include maximum permissible limits for chemical substances. Although the 1962 U.S. Public Health Service Drinking Water Standards were replaced in 1975 (effective in 1977) by national interim primary drinking water regulations, many of the original maximum permissible limits from 1962 were adopted as MCLs. MCLs are now periodically proposed or re-evaluated.
By law, MCLs are monitored on a prescribed schedule (frequency) and by using a specified analytical method. Legal violation of a MCL is not determined or based on the results of a single sample; it is based on a series of samples taken during the prescribed monitoring period.
Besides their primary use as regulatory standards for public water supplies, MCLs are useful in evaluating water quality data from private water supplies for determining potability. When applying MCLs to private water supplies, however, it is important to remember that they were developed considering more than just health concerns. MCLs are not intended to apply to single sample results, or to results from source water samples. To reasonably apply MCLs, data should originate from the MCL-specified analytical procedures.
References. EPA. 2000. Setting Standards for Safe Drinking Water. Office of Water. http://www.epa.gov/safewater/standard/setting.html. June 9, 2000.
EPA. 2001. Drinking Water Standards. Office of Water.
http://www.epa.gov/safewater/creg.html. July 23, 2001.
National Primary Drinking Water Regulations, 40 C.F.R. Sect. 141.1-141.210.
4.3 EPA Maximum Contaminant Level Goals (MCLGs), Drinking Water Equivalent Levels (DWELs), and Health Advisories (HAs)
Definition/Derivation. EPA establishes several guidelines for permissible levels of a substance in a drinking water supply, including maximum contaminant level goals (MCLGs), drinking water equivalent levels (DWELs), and health advisories (HAs). MCLGs, formerly known as Recommended Maximum Contaminant Levels, are drinking water health goals. MCLGs are set at a level at which EPA has found that "no known or anticipated adverse effect on human health occurs and which allows an adequate margin of safety." EPA considers the possible impact of synergistic effects, long-term and multi-stage exposures, and the existence of more susceptible groups in the population when determining MCLGs. For carcinogens, the MCLG is set at zero, unless data indicate otherwise, based on the assumption that there is no threshold for possible carcinogenic effects.
The DWEL is a lifetime exposure level specific for drinking water (assuming that all exposure is from drinking water) at which adverse, noncarcinogenic health effects would not be expected.
EPA developed HAs as substance concentrations in drinking water at which adverse noncarcinogenic health effects would not be anticipated with a margin of safety. Drinking water concentrations are developed to establish acceptable 1-day and 10-day exposure levels for both adults and children when toxicologic data (NOAEL or LOAEL) exist from animal or human studies. Short-term HAs are intended to be used for short-term exposures such as spills and accidents. Lifetime HAs represent that portion of an individual's total exposure to a chemical that is attributed to drinking water. This is considered protective of noncarcinogenic health effects occurring during a lifetime (70 years) of exposure. Lifetime HAs are derived from DWELs. For organic compounds, lifetime HAs are 20 % of the DWEL; for inorganic compounds, lifetime HAs are 10 % of the DWEL. Typically, lifetime HAs are not determined for class A and B carcinogens. When sufficient information is available, however, the substance concentration corresponding to a target cancer risk of 10-4 (an increase of one cancer case in a population of one thousand) may be calculated. For Class C carcinogens, the lifetime HA is divided by an additional factor of 10.
Applicability/Intended Use. MCLGs and Proposed Maximum Contaminant Level Goals (PMCLGs) are not legally enforceable values. However, SARA now requires attaining MCLGs when relevant and appropriate. MCLGs and PMCLGs are commonly used for developing and re-evaluating health advisories and are used as screening parameters for determining potability of private water supplies. MCLGs and PMCLGs may be more applicable than MCLs when identifying potable water supplies because they are strictly health-based.
DWELs are not legally enforceable, nor do they carry any legal authority under SDWA. However, they may be used as a source of information on noncarcinogenic health effects when developing or re-evaluating drinking water standards.
HAs are not legally enforceable standards, they are not issued as an official regulation, and they may or may not lead ultimately to the issuance of a national standard or MCL. Because MCLs consider occurrence, relative source contribution factors, treatment technologies, monitoring capability, costs, and health, it is more than likely that any resulting MCL would differ from the strictly health-based HA. The existence of an HA provides useful information to assist in setting control priorities in cases where contaminants in drinking water have been found.
References. Comprehensive Environmental Response, Compensation, and Liability Act of 1980, Pub. L. No. 95-510 (Dec 11, 1980), as amended by the Superfund Amendments and Reauthorization Act of 1986, Pub. L. No. 99-499 (Oct 17, 1986), codified together at 42 U.S.C. 9601, et seq.
EPA. 2000. Drinking Water Regulations and Health Advisories. Office of Water. Publication No.: EPA-822-B-00-001. http://www.epa.gov/ost/drinking/standards/. Summer 2000.
Note: Health advisory values may be re-evaluated and calculated without publishing new health advisory documents.
4.4 EPA Soil Screening Levels (SSLs)
Definition/Derivation. Soil screening levels (SSLs) are estimates of contaminant concentrations not expected to result in noncarcinogenic health effects during a specified duration of exposure (similar to EMEGs), or to be associated with no more than an estimated one excess cancer in a million (10-6) persons exposed during a 70 year life span (similar to CREGs). SSLs are derived by calculating exposure equations and pathway models to estimate an "acceptable" level of a contaminant in soil via ingestion, dermal, and inhalation pathways. SSLs combine EPA toxicity criteria with generic exposure parameters and are intended to be overly conservative and protective of most populations. SSLs also consider the potential of contaminants to migrate to groundwater, and are calculated such that substance migration to groundwater would meet MCLGs or MCLs.
Applicability/Intended Use. SSLs are used by EPA to help standardize and accelerate the evaluation and cleanup of contaminated soils at NPL sites by screening out areas, exposure pathways, or chemicals from further consideration. When contaminant concentrations fall below SSLs, no further action or study is necessary. Therefore, SSLs provide a means to focus resources on exposure areas, contaminants, and exposure pathways of potential concern. However, SSLs are not cleanup standards, and exceeding a SSL does not necessarily indicate an unacceptable level of substance in soil or the need for action. Generally, where contaminant concentrations exceed SSLs, EPA considers further study, not necessarily cleanup, to be necessary.
References. EPA. 1996. Soil Screening Guidance: Fact Sheet. Office of Emergency and Remedial Response. Publication No.: EPA/540/F-95/041. http://www.epa.gov/superfund/resources/soil/fact_sht.pdf. July 1996.
EPA. 1996. Soil Screening Guidance: User's Guide, Second Edition. Office of Emergency and Remedial Response. Publication No.: EPA/540/R-96/018. http://www.epa.gov/superfund/resources/soil/ssg496.pdf. July 1996.
4.5 EPA National Ambient Air Quality Standards (NAAQS)
Definition/Derivation. National Ambient Air Quality Standards (NAAQS) are set under Section 109 of the Clean Air Act (CAA) for any pollutants which, if present in air, might endanger the public health (primary standards) or public welfare (secondary standards). In developing primary standards, all sources of the pollutant that contribute to the health risk are considered. The standards must allow for an adequate margin of safety and must consider the nature and severity of the health effects of each contaminant, the most sensitive group of individuals at risk, and the degree of uncertainty of the scientific evidence. The CAA does not require EPA to consider economic or technical feasibility of implementing the standards.
Applicability/Intended Use. NAAQSs are not directly enforceable; they establish ceilings that should not be exceeded in an area where the source or sources of the pollutant are located. Thus, the standards determine restrictions on new sources and the degree of control to be imposed on existing sources. In effect, these controls determine if a new facility can be built in a given region and the type of pollution abatement systems that new and existing facilities must install. Standards can be promulgated as annual maximums, annual geometric means, annual arithmetic means, or for other time periods that vary from 1 hour to 1 year, depending on the pollutant.
References. Clean Air Act of 1970, as amended by the Clean Air Act Amendments of 1990, (November 15, 1990), 42 U.S.C. 7409. National ambient air quality standards. Sect. 109.
EPA. 2004. National Ambient Air Quality Standards (NAAQS). Office of Air Quality Planning and Standards. http://www.epa.gov/air/criteria.html. Last Updated October 1, 2004.
4.6 National Council on Radiation Protection and Measurements (NCRP) Radiation Guidelines and NCRP Soil Screening Limits
Definition/Derivation. The National Council on Radiation Protection and Measurements (NCRP) developed the radiation guidelines and soil screening limits as tools to aid in the cleanup of surface soil radionuclide contamination. The radiation guidelines and soil screening limits are derived by first reviewing the current models for estimating dose, then using the estimation in eight different land-use scenarios to calculate the highest annual exposure from external dose, or the committed effective dose from inhalation or ingestion that would be delivered by the radionuclide and its daughter products. Conservative values are selected to overestimate possible doses and to protect public health. This approach results in annual committed effective doses and screening limits that are realistic but still conservative.
Applicability/Intended Use. After ATSDR review, the Division of Health Assessment and Consultation (DHAC) adopted the use of NCRP Report 129 as a method of screening radiation levels in soil. Radiation guidelines and soil screening limits are used as a conservative method of relating an effective dose limit for an exposed critical population to a corresponding soil contamination level. Usually, these values are used for decision-making regarding the need for possible action based on present soil radionuclide levels. When radionuclide concentrations fall below the suggested limits, further action is generally not required. If the soil concentration exceeds the limit, then a site-specific dose assessment is recommended. The calculated doses are deliberately designed to conservatively represent the maximum dose to any individual. Therefore, these doses are inappropriate for use in calculating population exposures or for estimating health effects. The calculation of doses to actual individuals requires the use of site-specific and individual-specific parameters.
References. National Council on Radiation Protection and Measurements. 1999. Recommended Screening Limits for Contaminated Surface Soil and Review of Factors Relevant to Site-Specific Studies. NCRP Report No. 129. January 29, 1999.
4.7 Food and Drug Administration (FDA) Action Levels and Guidelines
Definition/Derivation. Action levels are enforceable regulatory limits of pesticides on or in human food, including fish, and animal feed. Food or feed may contain pesticide residues even if good agricultural or manufacturing practices were used. For example, some harmful substances persist in the environment. Action levels are derived considering the extent to which a pesticide cannot be avoided and existing analytical detection levels. In other words, action levels are not based exclusively on health considerations. The complete technical basis for the Food and Drug Administration (FDA) action levels is not publicly available. Action levels currently exist for approximately 23 toxic substances.
Tolerance levels were established by EPA as a measure of the maximum allowable levels of pesticide residues in or on raw agricultural products, including fish, and in processed food. If both a tolerance level and an action level exist for the same chemical or foodstuff, the tolerance level replaces the action level. Tolerance levels are derived by considering the possible toxic effects of a substance and the average daily intake of a food that contains the substance. A tolerance level is approved if the substance in a food is unlikely to result in an adverse health impact at the average daily intake rate.
Applicability/Intended Use. Action levels are used as legally enforceable guidance levels for pesticide residues when food additive regulations do not exist. If food, including fish, or feed exceeds the action level, the FDA has the discretion to take legal action to remove the product from the market.
Tolerance levels are used for testing food, including fish, and feed produce as soon as a food commodity is marketed so that any violations may be traced directly to the source. Tolerance levels are used to answer three questions: 1) what substance residues are in or on the foodstuff, 2) how much of the substance residues are in or on the foodstuff, and 3) is the level of dietary exposure to the substances acceptable. In other words, a tolerance level is the level at which no adverse effects would be expected to occur after a lifetime of dietary exposure to the substance under normal conditions.
References. FDA. 2000. Action Levels for Poisonous or Deleterious Substances in Human Food and Animal Feed. Industry Activities Staff. http://vm.cfsan.fda.gov/~lrd/fdaact.html. August 2000.
Federal Insecticide, Fungicide, and Rodenticide Act of 1976, as amended by the Food Quality Protection Act of 1996, Pub .L. No. 104-170 (August 3, 1996), 7 U.S.C. 136 et seq.
Food, Drug, and Cosmetic Act of 1938, as amended by the FDA Modernization Act of 1997, 21 U.S.C. 346, et seq.
Tolerances and Exemptions from Tolerances for Pesticide Chemicals in Food, 40 C.F.R. Sect. 180, et seq.
Unavoidable Contaminants in Food for Human Consumption and Food-packaging Material, 21 C.F.R. Sect. 109 (2000).
Unavoidable Contaminants in Animal Food and Food-packaging Material, 21 C.F.R. Sect. 509 (2000).
4.8 Occupational Safety and Health Administration (OSHA) Standards and Guidelines
Definition/Derivation. Permissible Exposure Limits (PELs) were developed by the Occupational Safety and Health Administration (OSHA) to provide safe and healthful working conditions, as mandated by Occupational Safety and Health Act of 1970. PELs are maximum exposure limits for certain airborne contaminants in the workplace, based on health criteria and technical feasibility. They are designed to ensure, to the extent feasible, that no employee suffers impairment of health or functional capacity even if regularly exposed to a substance throughout his/her working life.
PELs are usually listed as 8-hour time-weighted averages (TWA). The level may be exceeded at points in time, but the sum of the exposure levels averaged over 8 hours must not exceed the limit. In some cases, ceiling and peak levels are listed in place of, or in addition to, the 8-hour TWA. Ceiling values cannot be exceeded at any time. During a designated time period, substance concentrations may reach, but never exceed, a peak level.
The short-term exposure limit (STEL) is a 15-minute TWA which should not be exceeded at any time during a workday even if the 8-hour TWA is within the PEL. Exposures at the STEL should not exceed 15 minutes and should not be repeated more than four times per day. There should be at least a 60-minute interval between successive exposures at the STEL. A STEL is recommended only in cases in which toxic effects have been reported from high short-term exposures in either animals or humans. It is not a separate, independent exposure limit, but rather a supplement to the PEL.
Applicability/Intended Use. PELs and STELs are enforceable regulatory standards for contaminants in the workplace and are revised as new information becomes available. If an employee is exposed to an OSHA-regulated substance at a level exceeding the PEL or STEL, the employer must comply with the substance-specific health standards listed in 29 CFR part 1910 to reduce the exposure.
It is important to understand that PELs and STELs apply to healthy adult employees working 40-hour weeks and not to the general population—including children, the elderly, and the sick—who may be subject to continuous environmental exposure.
References. Air Contaminants, 29 C.F.R. Sect. 1910.1000, et seq. http://www.osha-slc.gov/SLTC/pel/index.html.
NIOSH. 2001. NIOSH Pocket Guide to Chemical Hazards and Other Databases. US Department of Health and Human Services. Publication No. 2001-145. http://www.cdc.gov/niosh/npg/npg.html. August 2001.
4.9 National Institute for Occupational Safety and Health (NIOSH) Recommended Exposure Limits (RELs)
Definition/Derivation. Under the authority of OSHA of 1970, the National Institute for Occupational Safety and Health (NIOSH) develops and periodically revises the recommended exposure limits (RELs), which are exposure limits for potentially hazardous substances or conditions in the workplace. NIOSH also publishes Immediately Dangerous to Life and Health (IDLH) levels, which represent the maximum concentration from which one could escape within 30 minutes without incurring impairing symptoms or irreversible health effects.
Applicability/Intended Use. RELs are available for airborne contaminants in the workplace. The RELs are developed as 8- or 10-hour TWAs or ceiling levels, as discussed under the definition and use of PELs. RELs are published and transmitted to OSHA and the Mine Safety and Health Administration for use in promulgating legal standards.
Similar to PELs and STELs, RELs apply to healthy adult employees working 40-hour weeks and not to the general population, who may be subject to continuous environmental exposure.
References. NIOSH. 2001. NIOSH Pocket Guide to Chemical Hazards and Other Databases. US Department of Health and Human Services. Publication No. 2001-145. http://www.cdc.gov/niosh/npg/npg.html. August 2001.
4.10 American Conference of Governmental Industrial Hygienists (ACGIH) Threshold Limit Values (TLVs)
Definition/Derivation. The American Conference of Governmental Industrial Hygienists (ACGIH) is an organization concerned with industrial health and occupational health and safety. With these concerns in mind, ACGIH has developed threshold limit values (TLVs), which are airborne concentrations of substances that are not believed to cause harmful effects in workers exposed regularly. ACGIH develops and updates TLVs based on toxicity information from industrial exposures, animal studies, and human studies, if available. ACGIH stresses that TLVs for individual substances may be based on different toxicologic studies and endpoints.
Applicability/Intended Use. TLVs are developed as a TWA for exposures 8 hours a day during a 40 hour work week and as TWA for short-term (15 minute) exposures, and as ceiling levels that should never be exceeded. TLVs are intended only as guidelines for protecting worker safety and do not represent an enforceable standard or finite level of toxicity.
Similar to OSHA and NIOSH values, TLVs apply to healthy adult employees working 40-hour weeks and not to the general population, who may be subject to continuous environmental exposure.
References. American Conference of Governmental Industrial Hygienists. http://www.acgih.org/home.htm.
References
ATSDR. 1993. Cancer Policy Framework. U.S. Department of Health and Human Services. January 1993.
ATSDR. 2001. Summary Report for the ATSDR Soil Pica Workshop. June 2000. Atlanta, Georgia. March 20, 2001.
Calabrese EJ and Stanek.EJ. 1998. Soil Ingestion estimation in children and adults: a dominant influence in site-specific risk assessment. Environmental News Reporter 28 ELR 10660-71. November 1998.
EPA. 1997. Exposure Factors Handbook. Volumes 1, 2, and 3. http://www.epa.gov/ncea/pdfs/efh/front.pdf
EPA. 2003. Draft final guidelines for carcinogen risk assessment final (external review draft). U.S. Environmental Protection Agency, Risk Assessment Forum, Washington, DC, NCEA-F-0644A. March 2003.
2 The framework for evaluating the evidence for nonlinear dose-response include asking questions such as: Is the hypothesized mode of action (MOA) sufficiently supported by test animals? Is the hypothesized MOA relevant to humans? Which populations or life stages can be particularly susceptible to the hypothesized MOA?
3 Because MRLs are subject to change, you should ensure that you are using EMEGs derived using the most up-to-date MRLs. The most current MRLs are available in the HazDat database or by reviewing the most current Toxicological Profile for a substance. For each example presented in this appendix, ATSDR has presented the most-up-to-date MRL at the time of publication.
4 ATSDR reports comparison values to one significant figure. Throughout this appendix, examples are reported to one significant figure.
5 A theoretical risk level is used to calculate CREGs because scientists employee a number of assumptions about the relative potency of a carcinogen at low doses. As such, the true risk is unknown and may be as low as zero (EPA 2003).
- Page last reviewed: November 30, 2005
- Page last updated: November 30, 2005
- Content source:



 ShareCompartir
ShareCompartir