Venetoclax
Venetoclax, sold under the trade name Venclexta and Venclyxto, is a medication used to treat chronic lymphocytic leukemia (CLL).[3]
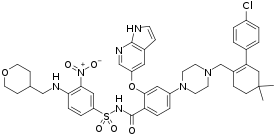 | |
| Clinical data | |
|---|---|
| Pronunciation | Venclexta /vɛnˈklɛkstə/ ven-KLEKS-tə |
| Trade names | Venclexta, Venclyxto |
| Other names | GDC-0199, ABT-199, RG7601[1] |
| AHFS/Drugs.com | venclexta |
| License data | |
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | >99.9%[2] |
| Metabolism | Liver (CYP3A4, CYP3A5) |
| Elimination half-life | ~26 hours |
| Excretion | Feces (>99.9%; 20.8% as unchanged venetoclax) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.254.611 |
| Chemical and physical data | |
| Formula | C45H50ClN7O7S |
| Molar mass | 868.44 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Medical uses
CLL/SLL
Venetoclax is used for adults with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).[3] Indication does not depend on mutation status (e. g. 17p deletion, IGHV mutation, 12+).
Other types of leukemia
Venetoclax is also used as part of a combination therapy for acute myeloid leukemia (AML).[3] For this purpose it is with azacitidine, decitabine, or low-dose cytarabine for newly-diagnosed adults over 75, or those with other health problems where intensive chemotherapy cannot be used.[3]
Side effects
Common side effects of venetoclax include neutropenia (low white blood cell count), nausea, anemia, diarrhea, upper respiratory tract infection, fatigue, and thrombocytopenia (low platelet count). Major side effects include tumor lysis syndrome and severe neutropenia. Additionally, this drug may cause fertility problems in males.[2]
Pharmacology
Mechanism of action
Venetoclax is a BH3-mimetic.[4] Venetoclax blocks the anti-apoptotic B-cell lymphoma-2 (Bcl-2) protein, leading to programmed cell death of CLL cells. Overexpression of Bcl-2 in some lymphoid malignancies has sometimes shown to be linked with increased resistance to chemotherapy.[5]
Pharmacokinetics
The maximum plasma concentration achieved after oral administration occurred 5-8 hours after dose.[2] Steady state maximum concentration with low-fat meal conditions at the 400 mg once daily dose was found to be 2.1 ± 1.1 μg/mL. It is recommended that Venetoclax be administered with a meal.[2]
The apparent volume of distribution for venetoclax is approximately 256–321 L. It is highly bound to human plasma protein. Within a concentration range of 1-30 μM (0.87-26 μg/mL), the fraction unbound in plasma was less than 0.01.[2]
Venetoclax is metabolized by CYP3A4/5 as proven by in-vitro studies.[2] Those using the drug should not consume grapefruit products because they contain CYP3A inhibitors.[2] Additionally, while using venetoclax it is not recommended to use other drugs which contain CYP3A inhibitors (i.e.: erythromycin, ciprofloxacin, diltiazem, dronedarone, fluconazole, verapamil).[2] Venetoclax is excreted from the body via the fecal route.[2]
History
In 2015, the United States Food and Drug Administration (FDA) granted the breakthrough therapy designation to venetoclax for people with CLL or SLL who have relapsed, become intolerant to, or refractory to previous treatment.
In 2016, the FDA approved venetoclax for use in those with CLL who have 17p deletion (deletion located on the chromosome 17 short arm) and who have been treated with at least one prior therapy.[2][6] Based on overall response rate, the indication was approved under accelerated FDA approval.[2]
In October 2016 a European Medicines Agency committee recommended conditional marketing approval for venetoclax for CLL in the presence of 17p deletion or TP53 mutation; the drug had already been granted orphan status in 2012 for that use.[7]
On June 8, 2018, the Food and Drug Administration granted regular approval to venetoclax for people with CLL or small lymphocytic lymphoma (SLL), with or without 17p deletion, who have received at least one prior therapy.[8] On May 15, 2019, the label has hence been extended by accelerated approval to include all adults with CLL/SLL disregarding prior treatment or mutation status.[3]
Society and culture
AbbVie Inc. of North Chicago Illinois manufactures Venclexta.[9] It is marketed by both Abbvie and Genentech USA, which is a member of the Roche Group.[9] AbbVie and Genentech are both commercializing the drug within the United States, but only AbbVie has rights to do so outside of the U.S.[10]
According to Reuters 2016 Drugs to Watch, the 2020 forecast sales for Venetoclax are 1.48 billion.[11] Competition as well as potential for combination is expected from other drugs such as ibrutinib and idelalisib, both of which were also approved in 2014 to treat CLL.[11][12]
Venclexta is patented by AbbVie Inc.[13]
Research
As of 2016 venetoclax had been tested to treat other hematological cancers, including non-Hodgkin’s lymphoma, multiple myeloma, diffuse large B-cell lymphoma and follicular lymphoma.[14]
References
- "Venetoclax". AdisInsight. Retrieved 21 November 2016.
- "US Venetoclax label" (PDF). FDA. April 2016.
- "FDA Prescribing Information" (PDF). FDA. Retrieved 5 June 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/208573s013lbl.pdf
- Roberts, Aw; Huang, Dcs (January 2017). "Targeting BCL2 With BH3 Mimetics: Basic Science and Clinical Application of Venetoclax in Chronic Lymphocytic Leukemia and Related B Cell Malignancies: Basic science and clinical application of venetoclax". Clinical Pharmacology & Therapeutics. 101 (1): 89–98. doi:10.1002/cpt.553. PMC 5657403. PMID 27806433.
- "Center for Drug Evaluation and Research - Application 208573Orig1s000 - Division Director Summary Review" (PDF). Food and Drug Administration (FDA). Retrieved 21 Nov 2016.
- "FDA Approves New Drug for Chronic Lymphocytic Leukemia in Patients with a Specific Chromosomal Abnormality". U.S. Food and Drug Administration. Retrieved 14 April 2016.
- "CHMP summary of positive opinion for Venclyxto". European Medicines Agency. Retrieved 21 November 2016.
- "FDA approves venetoclax for CLL or SLL, with or without 17p deletion, after one prior therapy". U.S. Food and Drug Administration.https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm610308.htm
- "Press Announcements - FDA approves new drug for chronic lymphocytic leukemia in patients with a specific chromosomal abnormality". www.fda.gov. Retrieved 2016-11-16.
- "Inside the development of Venclexta, AbbVie's new leukemia drug". BioPharma Dive. Retrieved 2016-11-16.
- "Drugs to Watch 2016 - Thomas Reuters" (PDF). Retrieved 16 Nov 2016.
- "Ibrutinib and Idelalisib Continue to Impress in CLL, May Eventually Replace Chemotherapy for Some Patients". OncLive. Retrieved 2016-11-17.
- "United States Patent: 9174982". patft.uspto.gov. Retrieved 2016-11-21.
- "Drugs to Watch 2016 - Market Insight Report" (PDF). Thomson Reuters. Retrieved 16 Nov 2016.