Selinexor
Selinexor (INN, trade name Xpovio; development code KPT-330) is a selective inhibitor of nuclear export used as an anti-cancer drug. It works by binding to exportin 1 and thus blocking the transport of several proteins involved in cancer-cell growth from the cell nucleus to the cytoplasm, which ultimately arrests the cell cycle and leads to apoptosis.[1] It is the first drug with this mechanism of action.[2][3]
 | |
| Clinical data | |
|---|---|
| Trade names | Xpovio |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619044 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 95% |
| Metabolism | Hepatic oxidation, glucuronidation, and conjugation, by CYP3A4, UGT and GST |
| Elimination half-life | 6–8 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| UNII | |
| Chemical and physical data | |
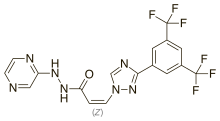
| Formula | C17H11F6N7O |
| Molar mass | 443.313 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Selinexor was granted accelerated approval by the U.S. Food and Drug Administration (FDA) in July 2019, for use in combination with the corticosteroid dexamethasone for the treatment of adult patients with relapsed refractory multiple myeloma (RRMM) who have received at least four prior therapies and whose disease is resistant to several other forms of treatment, including at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody.[4] In clinical trials, it was associated with a high incidence of severe side effects, including low platelet counts and low blood sodium levels.[3][5][6]
Medical uses
Selinexor is approved for use in combination with the steroid dexamethasone in people with relapsed or refractory multiple myeloma who have received at least four prior therapies and whose disease is refractory to at least two proteosome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody (so-called "quad-refractory" or "penta-refractory" myeloma),[7] for whom no other treatment options are available.[3][5] It is the first drug to be approved for this indication.[8]
Adverse effects
In the clinical study used to support FDA approval, selinexor was associated with high rates of pancytopenia, including leukopenia (28%), neutropenia (34%, severe in 21%), thrombocytopenia (74%, severe in 61% of patients), and anemia (59%).[5][9] The most common non-hematological side effects were gastrointestinal reactions (nausea, anorexia, vomiting, and diarrhea), hyponatremia (low blood sodium levels, occurring in up to 40% of patients), and fatigue.[9][10] More than half of all patients who received the drug developed infections, including fatal cases of sepsis.[9] However, these data are from an open-label trial, and thus cannot be compared to placebo or directly attributed to treatment.
Mechanism of action
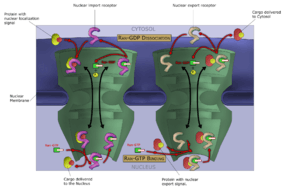
Like other selective inhibitors of nuclear export (SINEs), selinexor works by binding to exportin 1 (also known as XPO1 or CRM1). XPO1 is a karyopherin which performs nuclear transport of several proteins, including tumor suppressors, oncogenes, and proteins involved in governing cell growth, from the cell nucleus to the cytoplasm; it is often overexpressed and its function misregulated in several types of cancer.[1] By inhibiting the XPO1 protein, SINEs lead to a buildup of tumor suppressors in the nucleus of malignant cells and reduce levels of oncogene products which drive cell proliferation. This ultimately leads to cell cycle arrest and death of cancer cells by apoptosis.[1][2][9] In vitro, this effect appeared to spare normal (non-malignant) cells.[1][10]
Inhibiting XPO1 affects many different cells in the body which may explain the incidence of adverse reactions to selinexor.[2] Thrombocytopenia, for example, is a mechanistic and dose-dependent effect, occurring because selinexor causes a buildup of the transcription factor STAT3 in the nucleus of hematopoietic stem cells, preventing their differentiation into mature megakaryocytes (platelet-producing cells) and thus slowing production of new platelets.[2]
Chemistry
Selinexor is a fully synthetic small-molecule compound, developed by means of a structure-based drug design process known as induced-fit docking. It binds to a cysteine residue in the nuclear export signal groove of exportin 1. Although this bond is covalent, it is slowly reversible.[1]
History
Selinexor was developed by Karyopharm Therapeutics, a pharmaceutical company focused on the development of drugs that target nuclear transport. It was approved in the United States in July 2019,[4][11] on the basis of a single-arm Phase IIb clinical trial. The FDA decided to grant accelerated approval despite a previous recommendation from an FDA Advisory Committee Panel which had voted 8-5 to delay approving the drug until the results from an ongoing Phase III study were known.[3]
Selinexor in combination with dexamethasone was granted accelerated approval and was granted orphan drug designation.[4] The FDA granted the approval of Xpovio to Karyopharm Therapeutics.[4]
Research
Under the codename KPT-330, selinexor was tested in several preclinical animal models of cancer, including pancreatic cancer, breast cancer, non-small-cell lung cancer, lymphomas, and acute and chronic leukemias.[12] In humans, early clinical trials (phase I) have been conducted in non-Hodgkin lymphoma, blast crisis, and a wide range of advanced or refractory solid tumors, including colon cancer, head and neck cancer, melanoma, ovarian cancer, and prostate cancer.[12] Compassionate use in patients with acute myeloid leukemia has also been reported.[12]
The pivotal clinical trial which served to support approval of selinexor for people with relapsed/refractory multiple myeloma was an open-label study of 122 patients known as the STORM trial.[9] In all of the enrolled patients, patients had been treated with a median of seven prior treatment regimens including conventional chemotherapy, targeted therapy with bortezomib, carfilzomib, lenalidomide, pomalidomide, and a monoclonal antibody (daratumumab or isatuximab);[7] nearly all had also undergone hematopoietic stem cell transplantation but had disease that continued to progress.[9] The overall response rate was 26%, including two stringent complete responses; 39% of patients had a minimal response or better. The median duration of response was 4.4 months, median progression-free survival was 3.7 months, and median overall survival was 8.6 months.[13]
As of 2019, phase I/II and III trials are ongoing,[3][12][14] including the use of selinexor in other cancers and in combinations with other drugs used for multiple myeloma.[2]
References
- Fung HY, Chook YM (2014). "Atomic basis of CRM1-cargo recognition, release and inhibition". Semin Cancer Biol. 27: 52–61. doi:10.1016/j.semcancer.2014.03.002. PMC 4108548. PMID 24631835.
- Gandhi UH, Senapedis W, Baloglu E, Unger TJ, Chari A, Vogl D; et al. (2018). "Clinical implications of targeting XPO1-mediated nuclear export in multiple myeloma". Clin Lymphoma Myeloma Leuk. 18 (5): 335–345. doi:10.1016/j.clml.2018.03.003. PMID 29610030.CS1 maint: multiple names: authors list (link)
- Feuerstein, Adam (3 July 2019). "FDA approves new multiple myeloma drug despite toxicity concerns". STAT. Retrieved 6 July 2019.
- "FDA approves new treatment for refractory multiple myeloma". U.S. Food and Drug Administration (Press release). 3 July 2019. Archived from the original on 20 November 2019. Retrieved 20 November 2019.

- Mulcahy, Nick (3 July 2019). "FDA Approves Selinexor for Refractory Multiple Myeloma". Medscape. Retrieved 6 July 2019.
- "Drug Trials Snapshots: Xpovio". U.S. Food and Drug Administration (FDA). 16 July 2019. Archived from the original on 20 November 2019. Retrieved 20 November 2019.

- Chim CS, Kumar SK, Orlowski RZ, Cook G, Richardson PG, Gertz MA; et al. (2018). "Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond". Leukemia. 32 (2): 252–262. doi:10.1038/leu.2017.329. PMC 5808071. PMID 29257139.CS1 maint: multiple names: authors list (link)
- Barrett, Jennifer (3 July 2019). "New Treatment for Refractory Multiple Myeloma Granted FDA Approval". Pharmacy Times. Retrieved 7 July 2019.
- "Xpovio- selinexor tablet, film coated". DailyMed. 19 August 2019. Retrieved 20 November 2019.
- Chen C, Siegel D, Gutierrez M, Jacoby M, Hofmeister CC, Gabrail N (2018). "Safety and efficacy of selinexor in relapsed or refractory multiple myeloma and Waldenstrom macroglobulinemia". Blood. 131 (8): 855–863. doi:10.1182/blood-2017-08-797886. PMID 29203585.CS1 maint: multiple names: authors list (link)
- "Xpovio (selinexor) FDA Approval History". Drugs.com. 3 July 2019. Retrieved 20 November 2019.
- Parikh K, Cang S, Sekhri A, Liu D; et al. (2014). "Selective inhibitors of nuclear export (SINE)—a novel class of anti-cancer agents". J Hematol Oncol. 7: 78. doi:10.1186/s13045-014-0078-0. PMC 4200201. PMID 25316614.CS1 maint: multiple names: authors list (link)
- Jagannath, S. (22 August 2019). "Oral Selinexor–Dexamethasone for Triple-Class Refractory Multiple Myeloma". New England Journal of Medicine. 381 (8): 727–738. doi:10.1056/NEJMoa1903455. PMID 31433920.
- "KPT-330 OR Selinexor Clinical Trials". ClinicalTrials.gov. Retrieved 20 November 2019.
External links
- "Selinexor". Drug Information Portal. U.S. National Library of Medicine.
- "Drug Approval Package: Xpovio". U.S. Food and Drug Administration (FDA).</ref>