Samarium (153Sm) lexidronam
Samarium (153Sm) lexidronam (chemical name Samarium-153-ethylene diamine tetramethylene phosphonate, abbreviated Samarium-153 EDTMP, trade name Quadramet) is a chelated complex of a radioisotope of the element samarium with EDTMP. It is used to treat pain when cancer has spread to the bone.[1][2]
 | |
| Clinical data | |
|---|---|
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Identifiers | |
IUPAC name
| |
| PubChem CID | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
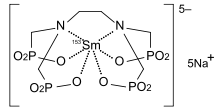
| Formula | C6H12N2Na5O12P4153Sm |
| Molar mass | 695.93 g/mol |
| 3D model (JSmol) |
|
SMILES
| |
It is injected into a vein and distributed throughout the body, where it is preferentially absorbed in areas where cancer has invaded the bone. The radioisotope 153Sm, with a half-life of 46.3 hours, decays by emitting beta particles (electrons), which kill the nearby cells. Pain begins to improve in the first week for most people and the effects can last several months. It is commonly used in lung cancer, prostate cancer, breast cancer, and osteosarcoma.
Side effects
Side effects[3] include the following:
- Black, tarry stools
- Blood in urine/stool
- Cough, hoarseness
- Fever/chills
- Lower back/side pain
- Painful or difficult urination
- Pinpoint red spots on skin
- Irregular heartbeat
- Nausea, vomiting
Supply and administration
Quadramet is supplied as a frozen solution for intravenous use with an activity of 50±5 mCi/mL[4] and beta energy 1986. Due to the short half-life of the radioisotope, the drug expires 56 hours after the noted calibration time.[4] Currently supported by Lantheus Medical Imaging.
References
- Anderson P (August 2006). "Samarium for osteoblastic bone metastases and osteosarcoma". Expert Opin Pharmacother. 7 (11): 1475–86. doi:10.1517/14656566.7.11.1475. PMID 16859431.
- Finlay IG; Mason MD; Shelley M (June 2005). "Radioisotopes for the palliation of metastatic bone cancer: a systematic review". Lancet Oncol. 6 (6): 392–400. doi:10.1016/S1470-2045(05)70206-0. PMID 15925817.
- "Quadramet Side Effects in Detail." Drugs.com
- "Quadramet Official FDA Information, side effects, and uses." Drugs.com, 2009.