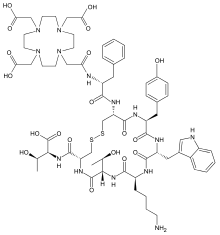
DOTA-TATE
{{Infobox drug | drug_name = Lutetium Lu 177 dotatate | INN = | type = | image = | width = | alt = | caption = | pronounce = | tradename = Lutathera | Drugs.com = Monograph | MedlinePlus = | licence_CA = | licence_EU = Yes | DailyMedID = Lutathera | licence_US = Lutathera | pregnancy_AU = | pregnancy_AU_comment = | pregnancy_US = N | pregnancy_US_comment = | pregnancy_category= | dependency_liability = | addiction_liability = | routes_of_administration = IV | class = Antineoplastic agents | ATCvet = | ATC_prefix = V10 | ATC_suffix = XX04 | ATC_supplemental = | legal_AU = | legal_AU_comment = | legal_BR = | legal_BR_comment = | legal_CA = | legal_CA_comment = | legal_DE = | legal_DE_comment = | legal_NZ = | legal_NZ_comment = | legal_UK = | legal_UK_comment = | legal_US = Rx-only | legal_US_comment = | legal_UN = | legal_UN_comment = | legal_status = | bioavailability = | protein_bound = | metabolism = | metabolites = | onset = | elimination_half-life = | duration_of_action = | excretion = | CAS_number = 437608-50-9 | CAS_supplemental = | PubChem = 71587735 | PubChemSubstance = | IUPHAR_ligand = | DrugBank = DB13985 | ChemSpiderID = | UNII = AE221IM3BB | KEGG = | ChEBI = | ChEMBL = | NIAID_ChemDB = | PDB_ligand = | synonyms = | IUPAC_name = (177Lu)lutetium(3+) 2-[4-({[(1R)-1-{[(4R,7S,10S,13R,16S,19R)-10-(4-aminobutyl)-4-{[(1S,2R)-1-carboxy-2-hydroxypropyl]-C-hydroxycarbonimidoyl}-6,9,12,15,18-pentahydroxy-7-[(1R)-1-hydroxyethyl]-13-[(1H-indol-3-yl)methyl]-16-[(4-oxidophenyl)methyl]-1,2-dithia-5,8,11,14,17-pentaazacycloicosa-5,8,11,14,17-pentaen-19-yl]-C-hydroxycarbonimidoyl}-2-phenylethyl]-C-hydroxycarbonimidoyl}methyl)-7,10-bis(carboxymethyl)-1,4,7,10-tetraazacyclododecan-1-yl]acetate | chemical_formula = | C = 65 | H = 87 | Lu = 1 | N = 14 | O = 19 | S = 2 | molecular_weight = | SMILES = CC(C1C(=O)NC(CSSCC(C(=O)NC(C(=O)NC(C(=O)NC(C(=O)N1)CCCCN)CC2=CNC3=CC=CC=C32)CC4=CC=C(C=C4)O)NC(=O)C(CC5=CC=CC=C5)NC(=O)CN6CCN(CCN(CCN(CC6)CC(=O)[O-])CC(=O)[O-])CC(=O)[O-])C(=O)NC(C(C)O)C(=O)O)O.[Lu+3] | Jmol = | StdInChI = 1S/C65H90N14O19S2.Lu/c1-38(80)56-64(96)73-51(63(95)75-57(39(2)81)65(97)98)37-100-99-36-50(72-59(91)47(28-40-10-4-3-5-11-40)68-52(83)32-76-20-22-77(33-53(84)85)24-26-79(35-55(88)89)27-25-78(23-21-76)34-54(86)87)62(94)70-48(29-41-15-17-43(82)18-16-41)60(92)71-49(30-42-31-67-45-13-7-6-12-44(42)45)61(93)69-46(58(90)74-56)14-8-9-19-66;/h3-7,10-13,15-18,31,38-39,46-51,56-57,67,80-82H,8-9,14,19-30,32-37,66H2,1-2H3,(H,68,83)(H,69,93)(H,70,94)(H,71,92)(H,72,91)(H,73,96)(H,74,90)(H,75,95)(H,84,85)(H,86,87)(H,88,89)(H,97,98);/q;+3/p-3/t38-,39-,46+,47-,48+,49-,50+,51+,56+,57+;/m1./s1/i;1+2 | StdInChI_comment = | StdInChIKey = MXDPZUIOZWKRAA-PRDSJKGBSA-K | density = | density_notes = | melting_point = | melting_high = | melting_notes = | boiling_point = | boiling_notes = | solubility = | sol_units = | specific_rotation = }}
 | |
| Names | |
|---|---|
| Other names
DOTA-(Tyr3)-octreotate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C65H90N14O19S2 |
| Molar mass | 1435.63 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
DOTA-TATE (DOTATATE,[1] DOTA-octreotate, oxodotreotide, DOTA-(Tyr3)-octreotate,[2] and DOTA-0-Tyr3-Octreotate) is an amino acid peptide, with a covalently bonded DOTA bifunctional chelator.
DOTA-TATE can be bound with radionuclides such as gallium-68 and lutetium-177 to form radiopharmaceuticals for PET imaging or radionuclide therapy. 177Lu DOTA-TATE therapy is a form of peptide receptor radionuclide therapy (PRRT) which targets somatostatin receptors (SSR).[3][4] In that form of application it is a form of targeted drug delivery.
Chemistry and mechanism of action
DOTA-TATE is a compound containing tyrosine3-octreotate,[2] an SSR agonist, and the bifunctional chelator DOTA (tetraxetan).[5][6] SSRs are found with high density in numerous malignancies, including CNS, breast, lung, and lymphatics.[7] The role of SSR agonists (i.e. somatostatin and its analogs such as octreotide, somatuline and vapreotide) in neuroendocrine tumours (NETs) is well established,[8] and massive SSR overexpression is present in several NETs. (Tyr3)-octreotate binds the transmembrane receptors of NETs with highest activity for SSR2 and is actively transported into the cell via endocytosis, allowing trapping of the radioactivity and increasing the probability of the desired double-strand DNA breakage (for tumour control). Trapping improves the probability of this kind of effect due to the relatively short range of the beta particles emitted by 177Lu, which have a maximum range in tissue of <2 mm.[9][8][10] Bystander effects include cellular damage by free radical formation.
Usage examples
68Ga DOTA-TATE (GaTate) is used to measure tumor SSR density and wholebody bio-distribution via PET imaging.[11][12] 68Ga DOTA-TATE imagery has a much higher sensitivity and resolution compared to 111In octreotide gamma camera or SPECT scans, due to intrinsic modality differences.[11]
177Lu DOTA-TATE, trade name Lutathera, was approved in the United States for the treatment of SSTR positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs), including foregut, midgut and hindgut neuroendocrine tumors in adults, in January 2018.[13][14][15][16] The recommended dosage is four cycles of 7.5 GBq every eight weeks. About four to six hours after infusion, the exposure rate of the patient has fallen to less than 25 μSv/hour at one metre and the patients can be discharged from hospital.[17]
Alternatives to 177Lu-DOTATE include yttrium-90 DOTATATE or DOTATOC. The larger range of 90Y may make it more suitable for large tumors with 177Lu reserved for smaller volumes[18][19]
History
Lutetium Lu 177
In January 2018, lutetium Lu 177 dotatate was approved in the United States for the treatment of a type of cancer that affects the pancreas or gastrointestinal tract called gastroenteropancreatic neuroendocrine tumors (GEP-NETs).[13] This was the first time a radioactive drug, or radiopharmaceutical, had been approved for the treatment of GEP-NETs.[13] Lutetium Lu 177 dotatate is indicated for adult patients with somatostatin receptor-positive GEP-NETs.[13]
The U.S. Food and Drug Administration (FDA) approved lutetium Lu 177 dotatate based primarily on evidence from one clinical trial, NETTER-1 (NCT01578239) of 229 patients with somatostatin-receptor positive midgut GEP-NETs.[15] Enrolled patients had tumors which could not be surgically removed and were worsening while receiving treatment with octreotide.[15] The trial was conducted at 41 sites in Belgium, France, Germany, Italy, Portugal, Spain, United Kingdom, and the United States.[15]
Patients were randomly assigned to receive either lutetium Lu 177 dotatate with long-acting octreotide or long-acting octreotide, at a higher dose, alone.[15] lutetium Lu 177 dotatate was injected through the vein and long-acting octreotide was injected in the muscle.[15] Both, patients and health care providers knew which treatment was given.[15] The benefit of lutetium Lu 177 dotatate was evaluated by measuring the length of time that tumors did not grow after treatment and compared it to the control group (progression free survival).[15]
The FDA considered additional data from a second study based on data from 1,214 patients with somatostatin receptor-positive tumors, including GEP-NETS, who received lutetium Lu 177 dotatate at a single site in the Netherlands, ERASMUS.[13][15] All patients received lutetium Lu 177 dotatate with octreotide.[15] Patients and health care providers knew which treatment was given.[15] The benefit of lutetium Lu 177 dotatate was evaluated by measuring if and how much the tumor size changed during treatment (the overall response rate).[15] Complete or partial tumor shrinkage was reported in 16 percent of a subset of 360 patients with GEP-NETs who were evaluated for response by the FDA.[13] Patients initially enrolled in the study received lutetium Lu 177 dotatate as part of an expanded access program.[13]
The application for lutetium Lu 177 dotatate was granted priority review designation and orphan drug designation.[13] The FDA granted the approval of Lutathera to Advanced Accelerator Applications.[13]
References
- Nockel, P. (2016). "The rate and clinical significance of incidental thyroid uptake as detected by gallium-68 DOTATATE positron emission tomography/computed tomography". Thyroid. 26 (6): 831–5. doi:10.1089/thy.2016.0174. PMC 4913484. PMID 27094616.
- Pubchem. "[Tyr3]octreotate". pubchem.ncbi.nlm.nih.gov. Retrieved 2 April 2018.
- Papotti, M.; Herder, W. W. de (2015). Neuroendocrine Tumors: A Multidisciplinary Approach. Karger Medical and Scientific Publishers. p. 77. ISBN 9783318027730.
- Aktolun, Cumali; Goldsmith, Stanley J. (2012). Nuclear Medicine Therapy: Principles and Clinical Applications. Springer. p. 364. ISBN 9781461440215.
- Pubchem. "Tetraxetan". pubchem.ncbi.nlm.nih.gov. Retrieved 2 April 2018.
- Fani, Melpomeni; Nicolas, Guillaume P.; Wild, Damian (1 September 2017). "Somatostatin Receptor Antagonists for Imaging and Therapy". Journal of Nuclear Medicine. 58 (Supplement 2): 61S–66S. doi:10.2967/jnumed.116.186783. PMID 28864614.
- Reubi JC, Laissue JA (March 1995). "Multiple actions of somatostatin in neoplastic disease". Trends in Pharmacological Sciences. 16 (3): 110–5. doi:10.1016/S0165-6147(00)88992-0. PMID 7792931.
- Mazziotti G, Mosca A, Frara S, Vitale G, Giustina A (November 2017). "Somatostatin analogs in the treatment of neuroendocrine tumors: current and emerging aspects". Expert Opinion on Pharmacotherapy. 18 (16): 1679–1689. doi:10.1080/14656566.2017.1391217. PMID 29067877.
- Emmett, Louise; Willowson, Kathy; Violet, John; Shin, Jane; Blanksby, Ashley; Lee, Jonathan (March 2017). "Lutetium177 PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy". Journal of Medical Radiation Sciences. 64 (1): 52–60. doi:10.1002/jmrs.227. PMC 5355374. PMID 28303694.
- Reubi JC, Schonbrunn A (December 2013). "Illuminating somatostatin analog action at neuroendocrine tumor receptors". Trends in Pharmacological Sciences. 34 (12): 676–88. doi:10.1016/j.tips.2013.10.001. PMC 3883302. PMID 24183675.
- Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ (February 2012). "High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours". Journal of Medical Imaging and Radiation Oncology. 56 (1): 40–7. doi:10.1111/j.1754-9485.2011.02327.x. PMID 22339744.
- Breeman WA, de Blois E, Sze Chan H, Konijnenberg M, Kwekkeboom DJ, Krenning EP (July 2011). "(68)Ga-labeled DOTA-peptides and (68)Ga-labeled radiopharmaceuticals for positron emission tomography: current status of research, clinical applications, and future perspectives". Seminars in Nuclear Medicine. 41 (4): 314–21. doi:10.1053/j.semnuclmed.2011.02.001. PMID 21624565.
- "FDA approves new treatment for certain digestive tract cancers". U.S. Food and Drug Administration (FDA) (Press release). 26 January 2018. Archived from the original on 11 December 2019. Retrieved 11 December 2019.

- "FDA approves lutetium Lu 177 dotatate for treatment of GEP-NETS". U.S. Food and Drug Administration (FDA) (Press release). 26 January 2018. Archived from the original on 11 December 2019. Retrieved 11 December 2019.

- "Drug Trials Snapshots: Lutathera". U.S. Food and Drug Administration (FDA). 20 February 2018. Archived from the original on 11 December 2019. Retrieved 11 December 2019.

- "Radiolabeled Peptide Offers PFS Benefit in Midgut NET". MedPage Today. 11 January 2017. Retrieved 25 April 201. Check date values in:
|accessdate=(help) - Calais, Phillipe J.; Turner, J. Harvey (1 April 2014). "Radiation safety of outpatient 177Lu-octreotate radiopeptide therapy of neuroendocrine tumors". Annals of Nuclear Medicine. 28 (6): 531–539. doi:10.1007/s12149-014-0843-8. PMID 24687907.
- Ramage, John K; Ahmed, A; Ardill, J; Bax, N; Breen, D J; Caplin, M E; Corrie, P; Davar, J; Davies, A H; Lewington, V; Meyer, T; Newell-Price, J; Poston, G; Reed, N; Rockall, A; Steward, W; Thakker, R V; Toubanakis, C; Valle, J; Verbeke, C; Grossman, A B (January 2012). "Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs)". Gut. 61 (1): 6–32. doi:10.1136/gutjnl-2011-300831. PMC 3280861. PMID 22052063.
- Bodei, L; Mueller-Brand, J; Baum, RP; Pavel, ME; Hörsch, D; O'Dorisio, MS; O'Dorisio, TM; Howe, JR; Cremonesi, M; Kwekkeboom, DJ; Zaknun, JJ (May 2013). "The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours". European Journal of Nuclear Medicine and Molecular Imaging. 40 (5): 800–16. doi:10.1007/s00259-012-2330-6. PMC 3622744. PMID 23389427.
- "European approval of lutetium oxodotreotide for gastroenteropancreatic neuroendocrine (GEP-NET) tumours". ecancer.org. 3 October 2017. Retrieved 2 April 2018.
- "Lutathera EPAR". European Medicines Agency (EMA). 4 February 2019. Archived from the original on 11 December 2019. Retrieved 11 December 2019.
External links
- "Lutetium Lu 177 dotatate". Drug Information Portal. U.S. National Library of Medicine.