Gallium scan
A gallium scan is a type of nuclear medicine test that uses either a gallium-67 (67Ga) or gallium-68 (68Ga) radiopharmaceutical to obtain images of a specific type of tissue, or disease state of tissue. Gallium salts like gallium citrate and gallium nitrate may be used. The form of salt is not important, since it is the freely dissolved gallium ion Ga3+ which is active.[1] Both 67Ga and 68Ga salts have similar uptake mechanisms.[2] Gallium can also be used in other forms, for example 68Ga-PSMA is used for cancer imaging. The gamma emission of gallium 67 is imaged by a gamma camera, while the positron emission of gallium 68 is imaged by positron emission tomography (PET).
| Gallium 67 scan | |
|---|---|
| Medical diagnostics | |
| Synonyms | Gallium imaging |
| ICD-10-PCS | C?1?LZZ (planar) C?2?LZZ (tomographic) |
| ICD-9-CM | 92.18 |
| OPS-301 code | 3-70c |
| MedlinePlus | 003450 |
Gallium salts are taken up by tumors, inflammation, and both acute and chronic infection,[3][4] allowing these pathological processes to be imaged. Gallium is particularly useful in imaging osteomyelitis that involves the spine, and in imaging older and chronic infections that may be the cause of a fever of unknown origin.[5][6]
Gallium citrate scan
.png)
In the past, the gallium scan was the gold standard for lymphoma staging, until it was replaced by positron emission tomography using fludeoxyglucose (FDG).[7][8] Gallium imaging is still used to image inflammation and chronic infections, and it still sometimes locates unsuspected tumors as it is taken up by many kinds of cancer cells in amounts that exceed those of normal tissues. Thus, an increased uptake of gallium-67 may indicate a new or old infection, an inflammatory focus from any cause, or a cancerous tumor.
It has been suggested that gallium imaging may become an obsolete technique, with indium leukocyte imaging and technetium antigranulocyte antibodies replacing it as a detection mechanism for infections. For detection of tumors, especially lymphomas, gallium imaging is still in use, but may be replaced by fludeoxyglucose PET imaging in the future.[9]
In infections, the gallium scan has an advantage over indium leukocyte imaging (also called indium-111 white blood cell scan) in imaging osteomyelitis (bone infection) of the spine, lung infections and inflammation, and for chronic infections. In part this is because gallium binds to neutrophil membranes, even after neutrophil death. Indium leukocyte imaging is better for acute infections (where neutrophils are still rapidly and actively localizing to the infection), and also for osteomyelitis that does not involve the spine, and for abdominal and pelvic infections. Both the gallium scan and indium leukocyte imaging may be used to image fever of unknown origin (elevated temperature without an explanation). However, the indium leukocyte scan will image only the 25% of such cases which are caused by acute infections, while gallium will also localize to other sources of fever, such as chronic infections and tumors.[10][11]
Mechanism
The body generally handles Ga3+ as though it were ferric iron (Fe-III), and thus the free isotope ion is bound (and concentrates) in areas of inflammation, such as an infection site, and also areas of rapid cell division.[12] Gallium (III) (Ga3+) binds to transferrin, leukocyte lactoferrin, bacterial siderophores, inflammatory proteins, and cell-membranes in neutrophils, both living and dead.[13]
Lactoferrin is contained within leukocytes. Gallium may bind to lactoferrin and be transported to sites of inflammation, or binds to lactoferrin released during bacterial phagocytosis at infection sites (and remains due to binding with macrophage receptors).[14] Ga-67 also attaches to the siderophore molecules of bacteria themselves, and for this reason can be used in leukopenic patients with bacterial infection (here it attaches directly to bacterial proteins, and leukocytes are not needed).[15] Uptake is thought to be associated with a range of tumour properties including transferring receptors, anaerobic tumor metabolism and tumor perfusion and vascular permeability.[16][17]
Common indications
- Whole-body survey to localize source of fever in patients with fever of unknown origin.[18]
- Detection of pulmonary and mediastinal inflammation/infection, especially in the immunocompromised patient.[19]
- Evaluation and follow-up of active lymphocytic or granulomatous inflammatory processes such as sarcoidosis or tuberculosis.[20]
- Diagnosing vertebral osteomyelitis and/or disk space infection where Ga-67 is preferred over labeled leukocytes.
- Diagnosis and follow-up of medical treatment of retroperitoneal fibrosis.
- Evaluation and follow-up of drug-induced pulmonary toxicity (e.g. Bleomycin, Amiodarone)
- Evaluation of patients who are not candidates for WBC scans (WBC count less than 6,000).
Note that all of these conditions are also seen in PET scans using the gallium-68.
Technique
The main (67Ga) technique uses scintigraphy to produce two-dimensional images. After the tracer has been injected, images are typically taken by a gamma camera at 24, 48, and in some cases, 72, and 96 hours later.[21][22] Each set of images takes 30–60 minutes, depending on the size of the area being imaged. The resulting image will have bright areas that collected large amounts of tracer, because inflammation is present or rapid cell division is occurring. Single photon emission computed tomography (SPECT) images may also be acquired. In some imaging centers, SPECT images may be combined with computed tomography scan using either fusion software or SPECT/CT hybrid cameras to superimpose both physiological image-information from the gallium scan, and anatomical information from the CT scan.
A common injection doses is around 150 megabecquerels.[23] Imaging should not usually be sooner than 24 hours - high background at this time produces false negatives. Forty-eight-hour whole body images are appropriate. Delayed imaging can be obtained even 1 week or longer after injection if bowel is confounding. SPECT can be performed as needed. Oral laxatives or enemas can be given before imaging to reduce bowel activity and reduce dose to large bowel; however, the usefulness of bowel preparation is controversial.[22]
10% to 25% of the dose of gallium-67 is excreted within 24 hours after injection (the majority of which is excreted through the kidneys). After 24 hours the principal excretory pathway is colon.[22] The "target organ" (organ that receives the largest radiation dose in the average scan) is the colon (large bowel).[21]
In a normal scan, uptake of gallium is seen in wide range of locations which do not indicate a positive finding. These typically include soft tissues, liver, and bone. Other sites of localisation can be nasopharyngeal and lacrimal glands, breasts (particularly in lactation or pregnancy), normally healing wounds, kidneys, bladder and colon.[24]
Gallium PSMA scan
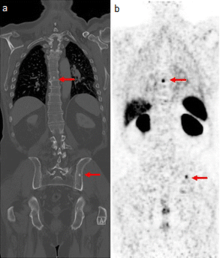
The positron emitting isotope, gallium 68, can be used to target prostate-specific membrane antigen (PSMA), a protein which is present in prostate cancer cells. The technique has been shown to improve detection of metastic disease compared to MRI or CT scans.[25]
Common indications
Gallium PSMA scanning is recommended primarily in cases of biochemical recurrence of prostate cancer, particularly for patients with low PSA values, and in patients with high risk disease where metastases are considered likely.[26][27]
Technique
An intravenous administration of 1.8–2.2 megabecquerels of 68Ga-PSMA per kilogram of bodyweight is recommended. Imaging should commence approximately 60 minutes after administration with an acquisition from mid-thigh to the base of the skull.[26][28]
Gallium DOTA scans
68Ga DOTA conjugated peptides (including 68Ga DOTATATE, DOTATOC and DOTANOC) are used for PET imaging of neuroendocrine tumours (NETs). The scan is similar to the SPECT octreotide scan in that a somatostatin analogue is used, and there are similar indications and uses, however image quality is significantly improved.[29] Somatostatin receptors are overexpressed in many NETs, so that the 68Ga DOTA conjugated peptide is preferentially taken up in these locations, and visualised on the scan.[30] As well as diagnosis and staging of NETs, 68Ga DOTA conjugated peptide imaging may be used for planning and dosimetry in preparation for lutetium-177 or yttrium-90 DOTA therapy.[31][32]
Radiochemistry of gallium-67
Gallium-67 citrate is produced by a cyclotron. Charged particle bombardment of enriched Zn-68 is used to produce gallium-67. The gallium-67 is then complexed with citric acid to form gallium citrate. The half life of gallium-67 is 78 hours.[33] It decays by electron capture, then emits de-excitation gamma rays that are detected by a gamma camera. Primary emission is at 93 keV (39% abundance), followed by 185 keV (21%) and 300 keV (17%).[34]:64 For imaging, multiple gamma camera energy windows are used, typically centred around 93 and 184 keV or 93, 184, and 296 keV.[22]
Radiochemistry of gallium-68
Gallium-68 is produced from decay of Germanium-68, which has a 270.8 day half-life.[35] Use of a generator means a supply of 68Ga can be produced easily with minimal infrastructure, for example at sites without a cyclotron, commonly used to produce other PET isotopes. It decays by positron emission and electron capture into Zinc-68.[36] Maximum energy of positron emission is at 1.9 MeV.[34]:65
References
- Treves, S. Ted (2014). Pediatric nuclear medicine and molecular imaging (4th ed.). Springer. p. 480. ISBN 9781461495512.
- Jain, Sanjay K. (2017). Imaging Infections: From Bench to Bedside. Springer. p. 34. ISBN 9783319545929.
- Verberne SJ and O. P. P. Temmerman (2017). 12 - Imaging of prosthetic joint infections - Arts, J.J. Chris. Management of Periprosthetic Joint Infections (PJIs). J. Geurts, Woodhead Publishing: 259-285.
- Verberne, SJ; Raijmakers, PG; Temmerman, OPP (2016). "The Accuracy of Imaging Techniques in the Assessment of Periprosthetic Hip Infection: A Systematic Review and Meta-Analysis". The Journal of Bone and Joint Surgery. American Volume. 98 (19): 1638–1645. doi:10.2106/jbjs.15.00898. PMID 27707850.
- Termaat, MF; Raijmakers, PG; Scholten, HJ; Bakker, FC; Patka, P; Haarman, HJ (November 2005). "The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis". The Journal of Bone and Joint Surgery. American Volume. 87 (11): 2464–71. doi:10.2106/JBJS.D.02691. PMID 16264122.
- Becker, W. (October 1995). "The contribution of nuclear medicine to the patient with infection". European Journal of Nuclear Medicine. 22 (10): 1195–1211. doi:10.1007/BF00800606. PMID 8542906.
- Bryan, R Nick (2010). Introduction to the science of medical imaging. Cambridge: Cambridge University Press. p. 200. ISBN 9780521747622.
- Bleeker-Rovers, C. P.; Vos, F. J.; van der Graaf, W. T. A.; Oyen, W. J. G. (16 June 2011). "Nuclear Medicine Imaging of Infection in Cancer Patients (With Emphasis on FDG-PET)". The Oncologist. 16 (7): 980–991. doi:10.1634/theoncologist.2010-0421. PMC 3228133. PMID 21680576.
- Ziessman, Harvey A.; O'Malley, Janis P.; Thrall, James H. (2013). Nuclear Medicine: The Requisites E-Book. Elsevier Health Sciences. p. 281. ISBN 978-0323112925.
- Palestro, Christopher J. (April 1994). "The current role of gallium imaging in infection". Seminars in Nuclear Medicine. 24 (2): 128–141. doi:10.1016/S0001-2998(05)80227-2. PMID 8023169.
- Shields, Thomas W.; LoCicero, Joseph; Reed, Carolyn E.; Feins, Richard H. (2009). General Thoracic Surgery. Lippincott Williams & Wilkins. p. 2106. ISBN 9780781779821.
- Love, C; Palestro, CJ (June 2004). "Radionuclide imaging of infection". Journal of Nuclear Medicine Technology. 32 (2): 47–57, quiz 58–9. PMID 15175400.
- Tsan, MF (January 1985). "Mechanism of gallium-67 accumulation in inflammatory lesions". Journal of Nuclear Medicine. 26 (1): 88–92. PMID 3880816.
- Greenberg, Alex M; Prein, Joachim (2007). Craniomaxillofacial reconstructive and corrective bone surgery principles of internal fixation using AO/ASIF technique. New York: Springer. p. 79. ISBN 9780387224275.
- Weiner, R.E. (1996). "The mechanism of 67Ga localization in malignant disease". Nuclear Medicine and Biology. 23 (6): 745–751. doi:10.1016/0969-8051(96)00119-9. PMID 8940716.
- Biersack, Hans-Jürgen; Freeman, Leonard M (2007). Clinical nuclear medicine. Berlin: Springer. p. 324. ISBN 978-3-540-28026-2.
- Hoffer, P (1980). "Gallium: mechanisms". Journal of Nuclear Medicine. 21 (3): 282–5. PMID 6988551.
- "Gallium scan". MedlinePlus. Retrieved 14 September 2017.
- "ACR–SPR Practice Parameter for the Performance of Scintigraphy for Inflammation and Infection" (PDF). American College of Radiology. 2014.
- "Lung gallium scan". MedlinePlus. Retrieved 14 September 2017.
- Bombardieri, Emilio; Aktolun, Cumali; Baum, Richard P.; Bishof-Delaloye, Angelica; Buscombe, John; Chatal, Jean François; Maffioli, Lorenzo; Moncayo, Roy; Mortelmans, Luc; Reske, Sven N. (2 September 2003). "67Ga Scintigraphy Procedure Guidelines for Tumour Imaging" (PDF). EANM.
- "Society of Nuclear Medicine Procedure Guideline for Gallium Scintigraphy in Inflammation" (PDF). SNMMI. 2 June 2004.
- "Notes for Guidance on the Clinical Administration of Radiopharmaceuticals and Use of Sealed Radioactive Sources" (PDF). Administration of Radioactive Substances Advisory Committee. January 2016. Retrieved 7 September 2016.
- Palestro, Christopher J. (2012). "SPECT and PET in the Assessment of Bone Infections". In Fogelman, Ignac; Gnanasegaran, Gopinath; van der Wall, Hans (eds.). Radionuclide and hybrid bone imaging. Berlin: Springer. pp. 523–559. doi:10.1007/978-3-642-02400-9_20. ISBN 978-3-642-02399-6.
- Maurer, Tobias; Eiber, Matthias; Schwaiger, Markus; Gschwend, Jürgen E. (23 February 2016). "Current use of PSMA–PET in prostate cancer management". Nature Reviews Urology. 13 (4): 226–235. doi:10.1038/nrurol.2016.26. PMID 26902337.
- Fendler, Wolfgang P.; Eiber, Matthias; Beheshti, Mohsen; Bomanji, Jamshed; Ceci, Francesco; Cho, Steven; Giesel, Frederik; Haberkorn, Uwe; Hope, Thomas A.; Kopka, Klaus; Krause, Bernd J.; Mottaghy, Felix M.; Schöder, Heiko; Sunderland, John; Wan, Simon; Wester, Hans-Jürgen; Fanti, Stefano; Herrmann, Ken (10 March 2017). "68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0". European Journal of Nuclear Medicine and Molecular Imaging. 44 (6): 1014–1024. doi:10.1007/s00259-017-3670-z. PMID 28283702.
- Rai, Bhavan Prasad; Baum, Richard Paul; Patel, Amit; Hughes, Robert; Alonzi, Roberto; Lane, Tim; Adshead, Jim; Vasdev, Nikhil (September 2016). "The Role of Positron Emission Tomography With 68Gallium (Ga)-Labeled Prostate-specific Membrane Antigen (PSMA) in the Management of Patients With Organ-confined and Locally Advanced Prostate Cancer Prior to Radical Treatment and After Radical Prostatectomy". Urology. 95: 11–15. doi:10.1016/j.urology.2015.12.048. PMID 26790588.
- Afaq, Asim; Batura, Deepak; Bomanji, Jamshed (14 February 2017). "New frontiers in prostate cancer imaging: clinical utility of prostate-specific membrane antigen positron emission tomography". International Urology and Nephrology. 49 (5): 803–810. doi:10.1007/s11255-017-1541-y. PMID 28197764.
- Mojtahedi, Alireza; Thamake, Sanjay; Tworowska, Izabela; Ranganathan, David; Delpassand, Ebrahim S (15 August 2014). "The value of 68Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature". American Journal of Nuclear Medicine and Molecular Imaging. 4 (5): 426–434. ISSN 2160-8407. PMC 4138137. PMID 25143861.
- Virgolini, Irene; Ambrosini, Valentina; Bomanji, Jamshed B.; Baum, Richard P.; Fanti, Stefano; Gabriel, Michael; Papathanasiou, Nikolaos D.; Pepe, Giovanna; Oyen, Wim; De Cristoforo, Clemens; Chiti, Arturo (2 July 2010). "Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE" (PDF). European Journal of Nuclear Medicine and Molecular Imaging. 37 (10): 2004–2010. doi:10.1007/s00259-010-1512-3. PMID 20596866.
- Kam, B. L. R.; Teunissen, J. J. M.; Krenning, E. P.; de Herder, W. W.; Khan, S.; van Vliet, E. I.; Kwekkeboom, D. J. (3 March 2012). "Lutetium-labelled peptides for therapy of neuroendocrine tumours". European Journal of Nuclear Medicine and Molecular Imaging. 39 (S1): 103–112. doi:10.1007/s00259-011-2039-y. PMC 3304065. PMID 22388631.
- Taïeb, David; Garrigue, Philippe; Bardiès, Manuel; Abdullah, Ahmad Esmaeel; Pacak, Karel (October 2015). "Application and Dosimetric Requirements for Gallium-68–labeled Somatostatin Analogues in Targeted Radionuclide Therapy for Gastroenteropancreatic Neuroendocrine Tumors". PET Clinics. 10 (4): 477–486. doi:10.1016/j.cpet.2015.06.001. PMC 4617555. PMID 26384594.
- IAEA (2009). Cyclotron produced radionuclides: physical characteristics and production methods (PDF). Vienna: International Atomic Energy Agency. p. 116. ISBN 9789201069085.
- Delacroix, D; Guerre, J P; Leblanc, P; Hickman, C (2002). Radionuclide and Radiation Protection Data Handbook (2nd ed.). Ashford: Nuclear Technology Publishing. ISBN 978-1870965873.
- Banerjee, Sangeeta Ray; Pomper, Martin G. (June 2013). "Clinical applications of Gallium-68". Applied Radiation and Isotopes. 76: 2–13. doi:10.1016/j.apradiso.2013.01.039. PMC 3664132. PMID 23522791.
- Bé, M M; Chisté, V; Mougeot, X; Chechev, V; Kondev, F; Nichols, A L; Huang, X; Wang, B (2013). Monographie BIPM: Table of radionuclides Vol. 7. Paris: Bureau International des Poids et Mesures. p. 33. ISBN 9789282222485.