Myeloproliferative neoplasm
The myeloproliferative neoplasms (MPNs), previously myeloproliferative diseases (MPDs), are a group of diseases of the bone marrow in which excess cells are produced. They are related to, and may evolve into, myelodysplastic syndrome and acute myeloid leukemia, although the myeloproliferative diseases on the whole have a much better prognosis than these conditions. The concept of myeloproliferative disease was first proposed in 1951 by the hematologist William Dameshek.[1] In the most recent World Health Organization classification of hematologic malignancies, this group of diseases was renamed from "myeloproliferative diseases" to "myeloproliferative neoplasms".[2] This reflects the underlying clonal genetic changes that are a salient feature of this group of disease.
| Myeloproliferative neoplasm | |
|---|---|
| Other names | Myeloproliferative diseases (MPDs) |
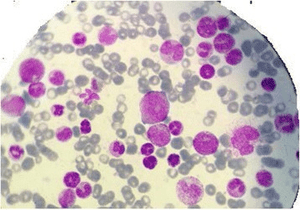 | |
| Myelogram of someone with a myeloproliferative disorder. | |
| Specialty | Hematology and oncology |
The increased numbers of blood cells may not cause any symptoms, but a number of medical problems or symptoms may occur. The risk of thrombosis is increased in some types of MPN.
Classification
Although a malignant neoplasm like other cancers, MPNs are classified within the hematological neoplasms. There are four main myeloproliferative diseases, which can be further categorized by the presence of the Philadelphia chromosome:
| Philadelphia chromosome positive | Philadelphia chromosome negative |
|---|---|
| |
In 2008, the World Health Organization listed these diagnoses as types of MPD:[3]
- Chronic myelogenous leukemia (BCR-ABL1–positive)
- Chronic neutrophilic leukemia
- Polycythemia vera
- Primary myelofibrosis
- Essential thrombocythemia
- Chronic eosinophilic leukemia (not otherwise specified)
- Mastocytosis
Causes
All MPNs arise from precursors of the myeloid lineages in the bone marrow. The lymphoid lineage may produce similar diseases, the lymphoproliferative disorders (acute lymphoblastic leukemia, lymphomas, chronic lymphocytic leukemia and multiple myeloma).
Most Philadelphia chromosome negative cases have an activating JAK2 or MPL mutation.[4] Mutations in CALR have been found in the majority of JAK2 and MPL-negative essential thrombocythemia and myelofibrosis.[5][6] In 2005, the discovery of the JAK2V617F mutation provided the first evidence that a fraction of persons with these disorders have a common molecular pathogenesis.[7][8][9][10] Patients with JAK2V617F-negative polycythemia vera are instead positive for another class of activating JAK2 mutations - the JAK2 exon 12 mutations.[11]
A subset may additionally have mutations in the genes LNK, CBL, TET2, ASXL1, IDH, IKZF1 or EZH2; the pathogenetic contribution of these mutations is being studied.
Diagnosis
Depending on the nature of the myeloproliferative neoplasm, diagnostic tests may include red cell mass determination (for polycythemia), bone marrow aspirate and trephine biopsy, arterial oxygen saturation and carboxyhaemoglobin level, neutrophil alkaline phosphatase level, vitamin B12 (or B12 binding capacity), serum urate[12] or direct sequencing of the patient's DNA.[13]
According to the WHO Classification of Hematopoietic and Lymphoid Neoplasms 2008 myeloproliferative neoplasms are divided into categories by diagnostic characteristics as follows:[3]
Chronic myeloid leukemia
Chronic myeloid leukemia (CML) with the defining translocation t(9;22);Philadelphia chromosome
Essential thrombocythemia
Essential thrombocythemia (ET) is associated with the JAK2 V617F mutation in up to 55% of cases[14] and with an MPL (thrombopoietin receptor) mutation in up to 5% of cases:[15]
- Cellular phase - increased large megakaryocytes with fibrosis and little increase in other bone marrow elements
- Fibrotic phase - collagenous fibrosis with lack of marrow elements
These disorders are still being revised according to more specific genetic mutations and how often patients end in a fibrotic marrow event.
Polycythemia vera
Polycythemia vera (PV) is associated most often with the JAK2 V617F mutation in greater than 95% of cases, whereas the remainder have a JAK2 exon 12 mutation:
- Cellular phase - increased megakaryocytes which cluster, reticulin fibrosis, later trichrome fibrosis, and increased myeloid and erythroid precursors
- Fibrotic phase - collagenous fibrosis with lack of marrow elements
Primary myelofibrosis
Primary myelofibrosis (PMF) is associated with the JAK2V617F mutation in up to 50% of cases, the JAK2 exon 12 mutations in 1-2% of cases, and the MPL (thrombopoietin receptor) mutation in up to 5% of cases:
- Prefibrotic/cellular phase - increased, small and atypical megakaryocytes which cluster, reticulin fibrosis, later trichrome (collagenous) fibrosis, and increased myeloid precursors
- Fibrotic phase - collagenous fibrosis with lack of marrow elements
Treatment
While investigational drug therapies exist, no curative drug treatment exists for any of the MPDs. The goal of treatment for ET and PV is prevention of thrombohemorrhagic complications. The goal of treatment for MF is amelioration of anemia, splenomegaly, and other symptoms. Low-dose aspirin is effective in PV and ET. Tyrosine kinase inhibitors like imatinib have improved the prognosis of CML patients to near-normal life expectancy.[4]
Recently, a JAK2 inhibitor, namely ruxolitinib, has been approved for use in primary myelofibrosis.[16] Trials of these inhibitors are in progress for the treatment of the other myeloproliferative neoplasms.
References
- Dameshek W (1951). "Some speculations on the myeloproliferative syndromes". Blood. 6 (4): 372–5. PMID 14820991.
- Tefferi A, Vainchenker W (February 2011). "Myeloproliferative neoplasms: molecular pathophysiology, essential clinical understanding, and treatment strategies". J. Clin. Oncol. 29 (5): 573–82. doi:10.1200/JCO.2010.29.8711. PMID 21220604.2
- Vardiman, J. W.; Thiele, J.; Arber, D. A.; Brunning, R. D.; Borowitz, M. J.; Porwit, A.; Harris, N. L.; Le Beau, M. M.; Hellström-Lindberg, E.; Tefferi, A.; Bloomfield, C. D. (2009). "The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes". Blood. 114 (5): 937–51. doi:10.1182/blood-2009-03-209262. PMID 19357394.
- Tefferi A, Vainchenker W (February 2011). "Myeloproliferative neoplasms: molecular pathophysiology, essential clinical understanding, and treatment strategies". J. Clin. Oncol. 29 (5): 573–82. doi:10.1200/JCO.2010.29.8711. PMID 21220604.
- Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379-2390
- Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391-2405
- Baxter EJ, Scott LM, Campbell PJ, et al. (2005). "Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders". Lancet. 365 (9464): 1054–1061. doi:10.1016/S0140-6736(05)71142-9. PMID 15781101.
- James C, Ugo V, Le Couedic JP, et al. (2005). "A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera". Nature. 434 (7037): 1144–1148. doi:10.1038/nature03546. PMID 15793561.
- Levine RL, Wadleigh M, Cools J, et al. (2005). "Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis". Cancer Cell. 7 (4): 387–397. doi:10.1016/j.ccr.2005.03.023. PMID 15837627.
- Kralovics R, Passamonti F, Buser AS, et al. (2005). "A gain-of-function mutation of JAK2 in myeloproliferative disorders". N Engl J Med. 352 (17): 1779–1790. doi:10.1056/NEJMoa051113. PMID 15858187.
- Scott LM, Tong W, Levine RL, et al. (2007). "JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis". N. Engl. J. Med. 356 (5): 459–68. doi:10.1056/NEJMoa065202. PMC 2873834. PMID 17267906.
- Levene, Malcolm I.; Lewis, S. M.; Bain, Barbara J.; Imelda Bates (2001). Dacie & Lewis Practical Haematology. London: W B Saunders. p. 586. ISBN 0-443-06377-X.
- Magor GW, Tallack MR, Klose NM, Taylor D, Korbie D, Mollee P, Trau M, Perkins AC (September 2016). "Rapid Molecular Profiling of Myeloproliferative Neoplasms Using Targeted Exon Resequencing of 86 Genes Involved in JAK-STAT Signaling and Epigenetic Regulation". The Journal of Molecular Diagnostics. 18 (5): 707–718. doi:10.1016/j.jmoldx.2016.05.006. PMID 27449473.
- Campbell PJ, Scott LM, Buck G, et al. (2005). "Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study". Lancet. 366 (9501): 1945–1953. doi:10.1016/S0140-6736(05)67785-9. PMID 16325696.
- Beer PA, Campbell PJ, Scott LM, Bench AJ, Erber WN, Bareford D, Wilkins BS, Reilly JT, Hasselbalch HC, Bowman R, Wheatley K, Buck G, Harrison CN, Green AR (July 2008). "MPL mutations in myeloproliferative disorders: analysis of the PT-1 cohort". Blood. 112 (1): 141–9. doi:10.1182/blood-2008-01-131664. PMID 18451306.
- Tibes R, Bogenberger JM, Benson KL, Mesa RA (October 2012). "Current outlook on molecular pathogenesis and treatment of myeloproliferative neoplasms". Mol Diagn Ther. 16 (5): 269–83. doi:10.1007/s40291-012-0006-3. PMID 23023734.
External links
| Classification |
|---|
- Myeloproliferative+Disorders at the US National Library of Medicine Medical Subject Headings (MeSH)
- MPN Cancer Connection
- MPN Research Foundation
- Myeloproliferative Disorders Website of The CMPD Education Foundation
- Myeloproliferative Disorders in practice
- Myeloproliferative Disorders Research Consortium
- MPN Info via Cancer.gov
- PV Reporter
- Spotlight on MPN