Glucagon (medication)
Glucagon is a medication and hormone.[1] As a medication it is used to treat low blood sugar, beta blocker overdose, calcium channel blocker overdose, and those with anaphylaxis who do not improve with epinephrine.[2] It is given by injection into a vein, muscle, or under the skin.[2]
 Glucagon ball and stick model, with the carboxyl terminus above and the amino terminus below | |
| Clinical data | |
|---|---|
| Trade names | GlucaGen, others |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | IV, IM, subQ |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C153H225N43O49S |
| Molar mass | 3482.747314 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Common side effects include vomiting.[2] Other side effects include low blood potassium and low blood pressure.[1] It is not recommended in people who have a pheochromocytoma or insulinoma.[2] Use in pregnancy has not be found to be harmful to the baby.[3] Glucagon is in the glycogenolytic family of medications.[2] It works by causing the liver to break down glycogen into glucose.[2]
Glucagon was approved for medical use in the United States in 1960.[2] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[4] The wholesale cost in the developing world is about US$25.75 a dose.[5] In the United Kingdom that dose costs the NHS about £11.52.[1] In the United States the wholesale cost of a dose is US$247.32.[6] It is a manufactured form of the glucagon hormone.[2]
Medical uses
Low blood sugar
An injectable form of glucagon may be part of first aid in cases of low blood sugar when the person is unconscious or for other reasons cannot take glucose orally or by intravenous. The glucagon is given by intramuscular, intravenous or subcutaneous injection, and quickly raises blood glucose levels. To use the injectable form, it must be reconstituted prior to use, a step that requires a sterile diluent to be injected into a vial containing powdered glucagon, because the hormone is highly unstable when dissolved in solution. When dissolved in a fluid state, glucagon can form amyloid fibrils, or tightly woven chains of proteins made up of the individual glucagon peptides, and once glucagon begins to fibrilize, it becomes useless when injected, as the glucagon cannot be absorbed and used by the body. The reconstitution process makes using glucagon cumbersome, although there are a number of products now in development from a number of companies that aim to make the product easier to use.
Beta blocker overdose
Anecdotal evidence suggests a benefit of higher doses of glucagon in the treatment of overdose with beta blockers; the likely mechanism of action is the increase of cAMP in the myocardium, in effect bypassing the β-adrenergic second messenger system.[7]
Anaphylaxis
Some people who have anaphylaxis and are on beta blockers are resistant to epinephrine. In this situation glucagon intravenously may be useful to treat their low blood pressure.[8]
Impacted food bolus
Glucagon relaxes the lower esophageal sphincter and may be used in those with an impacted food bolus in the esophagus ("steakhouse syndrome").[9] There is little evidence for glucagon's effectiveness in this condition,[10][11][12] and glucagon may induce nausea and vomiting,[12] but considering the safety of glucagon this is still considered an acceptable option as long it does not lead to delays in arranging other treatments.[13][14]
Endoscopic retrograde cholangiopancreatography
Glucagon's effect of increasing cAMP causes relaxation of splanchnic smooth muscle, allowing cannulation of the duodenum during the endoscopic retrograde cholangiopancreatography (ERCP) procedure.
Adverse effects
Glucagon acts very quickly; common side-effects include headache and nausea.
Drug interactions: Glucagon interacts only with oral anticoagulants, increasing the tendency to bleed.[15]
Contraindications
While glucagon can be used clinically to treat various forms of hypoglycemia, it is contraindicated in patients with pheochromocytoma, as it can induce the tumor to release catecholamines, leading to a sudden elevation in blood pressure.[16] Likewise, glucagon is contraindicated in patients with an insulinoma, as its hyperglycemic effect can induce the tumor to release insulin, leading to rebound hypoglycemia.[16]
Mechanism of action
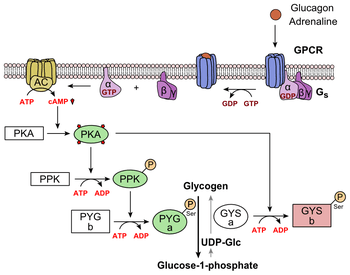
Glucagon binds to the glucagon receptor, a G protein-coupled receptor, located in the plasma membrane. The conformation change in the receptor activates G proteins, a heterotrimeric protein with α, β, and γ subunits. When the G protein interacts with the receptor, it undergoes a conformational change that results in the replacement of the GDP molecule that was bound to the α subunit with a GTP molecule. This substitution results in the releasing of the α subunit from the β and γ subunits. The alpha subunit specifically activates the next enzyme in the cascade, adenylate cyclase.
Adenylate cyclase manufactures cyclic adenosine monophosphate (cyclic AMP or cAMP), which activates protein kinase A (cAMP-dependent protein kinase). This enzyme, in turn, activates phosphorylase kinase, which then phosphorylates glycogen phosphorylase b, converting it into the active form called phosphorylase a. Phosphorylase a is the enzyme responsible for the release of glucose-1-phosphate from glycogen polymers.
Additionally, the coordinated control of glycolysis and gluconeogenesis in the liver is adjusted by the phosphorylation state of the enzymes that catalyze the formation of a potent activator of glycolysis called fructose-2,6-bisphosphate.[17] The enzyme protein kinase A that was stimulated by the cascade initiated by glucagon will also phosphorylate a single serine residue of the bifunctional polypeptide chain containing both the enzymes fructose-2,6-bisphosphatase and phosphofructokinase-2. This covalent phosphorylation initiated by glucagon activates the former and inhibits the latter. This regulates the reaction catalyzing fructose-2,6-bisphosphate (a potent activator of phosphofructokinase-1, the enzyme that is the primary regulatory step of glycolysis)[18] by slowing the rate of its formation, thereby inhibiting the flux of the glycolysis pathway and allowing gluconeogenesis to predominate. This process is reversible in the absence of glucagon (and thus, the presence of insulin).
Glucagon stimulation of PKA also inactivates the glycolytic enzyme pyruvate kinase.[19]
History
In the 1920s, Kimball and Murlin studied pancreatic extracts, and found an additional substance with hyperglycemic properties. They described glucagon in 1923.[20] The amino acid sequence of glucagon was described in the late 1950s.[21] A more complete understanding of its role in physiology and disease was not established until the 1970s, when a specific radioimmunoassay was developed.
References
- British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 487. ISBN 9780857111562.
- "Glucagon". The American Society of Health-System Pharmacists. Archived from the original on 26 December 2016. Retrieved 8 December 2016.
- "Glucagon (GlucaGen) Use During Pregnancy". www.drugs.com. Archived from the original on 27 December 2016. Retrieved 27 December 2016.
- "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- "Glucagon". International Drug Price Indicator Guide. Retrieved 8 December 2016.
- "NADAC as of 2016-12-21 | Data.Medicaid.gov". Centers for Medicare and Medicaid Services. Archived from the original on 24 December 2016. Retrieved 27 December 2016.
- White CM (May 1999). "A review of potential cardiovascular uses of intravenous glucagon administration". Journal of Clinical Pharmacology. 39 (5): 442–7. PMID 10234590.
- Tang AW (Oct 2003). "A practical guide to anaphylaxis". American Family Physician. 68 (7): 1325–32. PMID 14567487.
- Ko HH, Enns R (Oct 2008). "Review of food bolus management". Canadian Journal of Gastroenterology. 22 (10): 805–8. doi:10.1155/2008/682082. PMC 2661297. PMID 18925301.
- Arora S, Galich P (Mar 2009). "Myth: glucagon is an effective first-line therapy for esophageal foreign body impaction". Canadian Journal of Emergency Medicine. 11 (2): 169–71. doi:10.1017/s1481803500011143. PMID 19272219.
- Leopard D, Fishpool S, Winter S (Sep 2011). "The management of oesophageal soft food bolus obstruction: a systematic review". Annals of the Royal College of Surgeons of England. 93 (6): 441–4. doi:10.1308/003588411X588090. PMC 3369328. PMID 21929913.
- Weant KA, Weant MP (Apr 2012). "Safety and efficacy of glucagon for the relief of acute esophageal food impaction". American Journal of Health-System Pharmacy. 69 (7): 573–7. doi:10.2146/ajhp100587. PMID 22441787.
- Ikenberry SO, Jue TL, Anderson MA, Appalaneni V, Banerjee S, Ben-Menachem T, Decker GA, Fanelli RD, Fisher LR, Fukami N, Harrison ME, Jain R, Khan KM, Krinsky ML, Maple JT, Sharaf R, Strohmeyer L, Dominitz JA (Jun 2011). "Management of ingested foreign bodies and food impactions" (PDF). Gastrointestinal Endoscopy. 73 (6): 1085–1091. doi:10.1016/j.gie.2010.11.010. PMID 21628009. Archived (PDF) from the original on 2013-08-08.
- Chauvin A, Viala J, Marteau P, Hermann P, Dray X (Jul 2013). "Management and endoscopic techniques for digestive foreign body and food bolus impaction". Digestive and Liver Disease. 45 (7): 529–42. doi:10.1016/j.dld.2012.11.002. PMID 23266207.
- Koch-Weser J (Mar 1970). "Potentiation by glucagon of the hypoprothrombinemic action of warfarin". Annals of Internal Medicine. 72 (3): 331–5. doi:10.7326/0003-4819-72-3-331. PMID 5415418.
- "Information for the Physician: Glucagon for Injection (rDNA origin)" (PDF). Eli Lilly and Company. Archived (PDF) from the original on 2016-11-20. Retrieved 2016-10-12.
- Hue L, Rider MH (Jul 1987). "Role of fructose 2,6-bisphosphate in the control of glycolysis in mammalian tissues". The Biochemical Journal. 245 (2): 313–24. doi:10.1042/bj2450313. PMC 1148124. PMID 2822019.
- Claus TH, El-Maghrabi MR, Regen DM, Stewart HB, McGrane M, Kountz PD, Nyfeler F, Pilkis J, Pilkis SJ (1984). The role of fructose 2,6-bisphosphate in the regulation of carbohydrate metabolism. Current Topics in Cellular Regulation. 23. pp. 57–86. doi:10.1016/b978-0-12-152823-2.50006-4. ISBN 9780121528232. PMID 6327193.
- Feliú JE, Hue L, Hers HG (Aug 1976). "Hormonal control of pyruvate kinase activity and of gluconeogenesis in isolated hepatocytes". Proceedings of the National Academy of Sciences of the United States of America. 73 (8): 2762–6. Bibcode:1976PNAS...73.2762F. doi:10.1073/pnas.73.8.2762. PMC 430732. PMID 183209.
- Kimball C, Murlin J (1923). "Aqueous extracts of pancreas III. Some precipitation reactions of insulin". J. Biol. Chem. 58 (1): 337–348. Archived from the original on 2007-09-29.
- Bromer W, Winn L, Behrens O (1957). "The amino acid sequence of glucagon V. Location of amide groups, acid degradation studies and summary of sequential evidence". J. Am. Chem. Soc. 79 (11): 2807–2810. doi:10.1021/ja01568a038.