Dibenzofuran
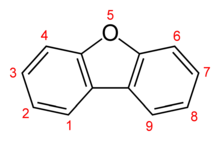
Dibenzofuran is a heterocyclic organic compound with the chemical structure shown at right. It is an aromatic compound that has two benzene rings fused to a central furan ring. All the numbered carbon atoms have a hydrogen atom bonded to each of them. It is a volatile white solid that is soluble in nonpolar organic solvents. It is obtained from coal tar, where it exists as a 1% component.[1]
 | |
 | |
| Identifiers | |
|---|---|
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.612 |
| KEGG | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C12H8O |
| Molar mass | 168.19 g/mol |
| Appearance | white crystalline powder |
| Melting point | 81 to 85 °C (178 to 185 °F; 354 to 358 K) |
| Boiling point | 285 °C (545 °F; 558 K) |
Solubility in water |
Insoluble |
| Hazards | |
| R-phrases (outdated) | R51/53 |
| S-phrases (outdated) | S24/25 S29 S61 |
| Related compounds | |
Related compounds |
Furan Benzofuran Dibenzodioxin Dibenzothiophene Carbazole Polyozellin (compound with a kernel with two dibenzofurans that share the same benzene ring) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Reactions
Dibenzofuran is thermally robust with a convenient liquid range. These properties, together with its low toxicity, are exploited by the use of DBF as a heat transfer agent.[1]
It undergoes electrophilic reactions, such as halogenation and Friedel-Crafts reactions. Reaction of DBF with butyl lithium results in dilithiation.[2]
Dibenzofuran is the precursor to the drug furobufen by Friedel-Crafts reaction with succinic anhydride.
Safety
Dibenzofuran is a relatively non-toxic compound as evidenced by rats being unaffected after a 200-day diet consisting of 0.025 – 0.4% of DBF.[1] The polychlorinated dibenzofurans are however controversial and potentially dangerous.
See also
- Benzofuran
References
- Gerd Collin and Hartmut Höke "Benzofurans" in Ullmann's Encyclopedia of Industrial Chemistry, 2007, Wiley-VCH, Weinheim. doi:10.1002/14356007.l03_l01
- Ulrich Iserloh, Yoji Oderaotoshi, Shuji Kanemasa, and Dennis P. Curran "Synthesis of (R,R)-4,6-Dibenzofurandiyl-2,2'-Bis (4-Phenyloxazoline) (DBFOX/PH) – A Novel Trridentate Ligand" Org. Synth. 2003, volume 80, 46. doi:10.15227/orgsyn.080.0046