22R-Hydroxycholesterol
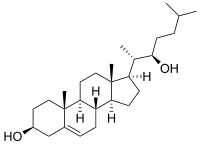
22R-Hydroxycholesterol, or (3β)-cholest-5-ene-3,22-diol is an endogenous, metabolic intermediate in the biosynthesis of the steroid hormones from cholesterol.[1][2] Cholesterol ((3β)-cholest-5-en-3-ol) is hydroxylated by cholesterol side-chain cleavage enzyme (P450scc) to form 22R-hydroxycholesterol, which is subsequently hydroxylated again by P450scc to form 20α,22R-dihydroxycholesterol, and finally the bond between carbons 20 and 22 is cleaved by P450scc to form pregnenolone ((3β)-3-hydroxypregn-5-en-20-one),[1][2] the precursor to the steroid hormones.
 | |
| Names | |
|---|---|
| IUPAC name
(3β)-Cholest-5-ene-3,22-diol | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C27H46O2 |
| Molar mass | 402.653 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is an agonist of the liver X receptor.
See also
- 20α,22R-Dihydroxycholesterol
- 27-Hydroxycholesterol
References
- CHAUDHURI AC, HARADA Y, SHIMIZU K, GUT M, DORFMAN RI (March 1962). "Biosynthesis of pregnenolone from 22-hydroxycholesterol". The Journal of Biological Chemistry. 237: 703–4. PMID 13878470.
- Hume R, Kelly RW, Taylor PL, Boyd GS (May 1984). "The catalytic cycle of cytochrome P-450scc and intermediates in the conversion of cholesterol to pregnenolone". European Journal of Biochemistry / FEBS. 140 (3): 583–91. doi:10.1111/j.1432-1033.1984.tb08142.x. PMID 6723652.
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.