ALK inhibitor
ALK inhibitors are potential anti-cancer drugs that act on tumours with variations of anaplastic lymphoma kinase (ALK) such as an EML4-ALK translocation.[1]
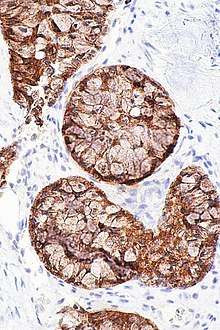
Micrograph showing an ALK positive adenocarcinoma of the lung. The ALK immunostain allows individuals with ALK rearrangements to be identified.
EML4-ALK
About 4-7% of non-small cell lung carcinomas (NSCLC) have EML4-ALK translocations.[2]
Approved inhibitors
- crizotinib (also a ROS1 inhibitor) approved in Aug 2011 by the US FDA for ALK-positive NSCLC.[3]
- ceritinib, approved by the FDA in April 2014 for treatment of NSCLC.[2][4]
- alectinib FDA approved Dec 2015 (accelerated), full approval in 2017 for ALK-positive NSCLC.
- brigatinib (also an EGFR inhibitor) approved in April 2017 by the US FDA for ALK-positive NSCLC.[5]
- lorlatinib approved in 2018 by the US FDA for ALK-positive NSCLC.
Clinical trials
Additional ALK inhibitors currently (or soon to be) undergoing clinical trials include:
- Entrectinib (Nerviano's NMS-E628, licensed by Ignyta and renamed RXDX-101, in the U.S. orphan drug designation and rare pediatric disease designation for the treatment of neuroblastoma and orphan drug designation for treatment of TrkA-, TrkB-, TrkC-, ROS1- and ALK-positive NSCLC)
- TSR-011 (Tesaro)
- CEP-37440 (Teva)
- X-396 (Xcovery)
Discontinued
- ASP-3026 (Astellas)
NPM-ALK
NPM-ALK is a different variation/fusion of ALK that drives anaplastic large-cell lymphomas (ALCLs) and is the target of other ALK inhibitors.[6] [7]
References
- Nelsen (2010). "ALK Inhibitors: Possible New Treatment for Lung Cancer".
- Farmer (2010). "Non-Small-Cell Lung Cancer Standards of Care Challenged by a Cornucopia of New Drugs".
- Chustecka (2010). "Crizotinib in ALK-NSCLC; Response Rate "Unprecedented"".
- "FDA Approves Ceritinib for ALK-Positive Lung Cancer". Medscape. April 29, 2014.
- https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/ucm537040.htm
- Galkin; et al. (2007). "Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK".
- "Archived copy". Archived from the original on 2010-12-23. Retrieved 2010-10-02.CS1 maint: archived copy as title (link)
External links
This article is issued from
Wikipedia.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.