Chloropicrin
Chloropicrin, also known as PS and nitrochloroform, is a chemical compound currently used as a broad-spectrum antimicrobial, fungicide, herbicide, insecticide, and nematicide.[4] Its chemical structural formula is Cl3CNO2.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
trichloro(nitro)methane | |||
| Other names
PS, Nitrochloroform, Trichloronitromethane | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
Beilstein Reference |
1756135 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.847 | ||
| EC Number |
| ||
Gmelin Reference |
240197 | ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1580 | ||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
CCl3NO2 | ||
| Molar mass | 164.375 g/mol | ||
| Appearance | colorless liquid | ||
| Odor | irritating[1] | ||
| Density | 1.692 g/ml[2] | ||
| Melting point | −69 °C (−92 °F; 204 K) | ||
| Boiling point | 112 °C (234 °F; 385 K) (decomposes) | ||
Solubility in water |
0.2%[1] | ||
| Vapor pressure | 18 mmHg (20°C) [1] | ||
Magnetic susceptibility (χ) |
-75.3·10−6 cm3/mol | ||
| Hazards | |||
| Main hazards | Extremely toxic and irritating to skin, eyes, and lungs. | ||
| GHS pictograms | 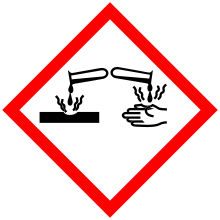    | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H301, H314, H318, H330, H370, H372, H400, H410 | ||
GHS precautionary statements |
P260, P264, P270, P271, P273, P280, P284, P301+310, P301+330+331, P303+361+353, P304+340, P305+351+338, P307+311, P310, P314, P320, P321, P330, P363, P391, P403+233, P405, P501 | ||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration) |
9.7 ppm (mouse, 4 hr) 117 ppm (rat, 20 min) 14.4 ppm (rat, 4 hr)[3] | ||
LCLo (lowest published) |
293 ppm (human, 10 min) 340 ppm (mouse, 1 min) 117 ppm (cat, 20 min)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 0.1 ppm (0.7 mg/m3)[1] | ||
REL (Recommended) |
TWA 0.1 ppm (0.7 mg/m3)[1] | ||
IDLH (Immediate danger) |
2 ppm[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Synthesis
Chloropicrin was discovered in 1848 by Scottish chemist John Stenhouse. He prepared it by the reaction of sodium hypochlorite with picric acid:
- HOC6H2(NO2)3 + 11 NaOCl → 3 Cl3CNO2 + 3 Na2CO3 + 3 NaOH + 2 NaCl
Because of the precursor used, Stenhouse named the compound chloropicrin, although the two compounds are structurally dissimilar.
Today, chloropicrin is manufactured by the reaction of nitromethane with sodium hypochlorite:[5]
- H3CNO2 + 3 NaOCl → Cl3CNO2 + 3 NaOH
or by the reaction of chloroform with nitric acid
CHCl3 + HNO3 → CCl3NO2 + H2O
Properties
Chloropicrin's chemical formula is CCl3NO2 and its molecular weight is 164.38 grams/mole.[6] Pure chloropicrin is a colorless liquid, with a boiling point of 112 °C.[6] Chloropicrin is sparingly soluble in water with solubility of 2000 mg/L at 25 °C.[6] It is volatile, with a vapor pressure of 23.2 millimeters of mercury (mmHg) at 25 °C; the corresponding Henry's law constant is 0.00251 atmosphere-cubic meter per mole.[6] The octanol-water partition coefficient (Kow) of chloropicrin is estimated to be 269.[6] Its soil adsorption coefficient (Koc; normalized to soil organic matter content) is 25 cm3/g.[6]
Use
Chloropicrin was manufactured for use as poison gas in World War I.[7] In agriculture, chloropicrin is injected into soil prior to planting a crop in order to fumigate soil. Chloropicrin affects a broad spectrum of fungi, microbes, insects.[8][9] It is commonly used as a stand-alone treatment or in combination / co-formulation with methyl bromide and 1,3-Dichloropropene.[9][10] Chloropicrin is used as an indicator and repellent while fumigating residences for insects with sulfuryl fluoride which is an odorless gas.[11]
Safety
At the national level, chloropicrin is regulated by the United States Environmental Protection Agency as a restricted use pesticide.[8][12] Because of its toxicity and carcinogenicity, distribution and use of chloropicrin is available only to licensed professionals and specially certified growers who are trained in its proper and safe use.[8][12] In the US, occupational exposure limits have been set at 0.1 ppm over an eight-hour time-weighted average.[13]
Agriculture
In 2008 the US EPA re-approved chloropicrin as safe for use in agricultural settings, stating that treatments "can provide benefits to both food consumers and growers. For consumers, it means more fresh fruits and vegetables can be cheaply produced domestically year-round because several severe pest problems can be efficiently controlled."[14][15][16] To ensure chloropicrin is used safely, the EPA requires a strict set of protections for handlers, workers, and persons living and working in and around farmland during treatments.[8][15] EPA protections were increased in both 2011 and 2012, reducing fumigant exposures and significantly improving safety.[17] Protections include the training of certified applicators supervising pesticide application, the use of buffer zones, posting before and during pesticide application, fumigant management plans, and compliance assistance and assurance measures.[16][18]
Used as a preplant soil treatment measure, chloropicrin suppresses soilborne pathogenic fungi and some nematodes and insects. With a half-life of hours to days, it is completely digested by soil organisms before the crop is planted, making it safe and efficient. Contrary to popular belief, chloropicrin does not sterilize soil and does not deplete the ozone layer, as the compound is destroyed by sunlight. Additionally, chloropicrin has never been found in groundwater, due to its low solubility. [19]
California
In California experience with acute effects of chloropicrin when used as a soil fumigant for strawberries and other crops led to the release of regulations in January 2015 creating buffer zones and other precautions to minimize exposure of farm workers, neighbors, and passersby.[20][21]
High Concentrations
Chloropicrin is harmful to humans. It can be absorbed systemically through inhalation, ingestion, and the skin. At high concentrations it is severely irritating to the lungs, eyes, and skin.[22] In World War I German forces used concentrated chloropicrin against Allied forces as a tear gas. While not as lethal as other chemical weapons, it caused vomiting and forced Allied soldiers to remove their masks to vomit, exposing them to other, more toxic chemical gases used as weapons during the war.[23]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0132". National Institute for Occupational Safety and Health (NIOSH).
- http://msds.chem.ox.ac.uk/CH/chloropicrin.html
- "Chloropicrin". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- "RED Fact Sheet: Chloropicrin" (PDF). US Environmental Protection Agency. 10 July 2008. p. 2. Retrieved 20 September 2013.
- Sheldon B. Markofsky "Nitro Compounds, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
- "Executive Summary - Evaluation of Chloropicrin As A Toxic Air Contaminant" (PDF). Department of Pesticide Regulation - California Environmental Protection Agency. February 2010. p. iv. Archived from the original (PDF) on 6 October 2013. Retrieved 20 September 2013.
- Ayres, Leonard P. (1919). The War with Germany (Second ed.). Washington, DC: United States Government Printing Office. p. 80.
- "Chloropicrin - Background". Retrieved 20 September 2013.
- http://www.workcover.nsw.gov.au/Documents/Publications/AlertsGuidesHazards/DangerousGoodsExplosivesFireworksPyrotechnics/chloropicrin_fact_sheet_1371.pdf Archived May 20, 2009, at the Wayback Machine
- Amos Alfred Fries; Clarence Jay West (1921). Chemical warfare. McGraw-Hill book company, inc. p. 144.
- http://termitetenting.com/
- "Chloropicrin Mitigation Proposal" (PDF). Department of Pesticide Regulation - California Environmental Protection Agency. 15 May 2013. p. 1. Archived from the original (PDF) on 6 October 2013. Retrieved 20 September 2013.
- "NIOSH Pocket Guide to Chemical Hazards". Centers for Disease Control and Prevention. Retrieved 7 November 2013.
- "Amended Reregistration Eligibility Decision (RED) for Chloropicrin" (PDF). United States Environmental Protection Agency. May 2009. Retrieved 20 September 2013.
- "Chloropicrin Mitigation Proposal" (PDF). Department of Pesticide Regulation - California Environmental Protection Agency. 15 May 2013. p. 2. Archived from the original (PDF) on 6 October 2013. Retrieved 20 September 2013.
- "Environmental Protection Agency (EPA) Re-Registers Chloropicrin As Safe For Use Nationwide". Retrieved 22 October 2013.
- "Chloropicrin Mitigation Proposal" (PDF). Department of Pesticide Regulation - California Environmental Protection Agency. 15 May 2013. p. 2. Archived from the original (PDF) on 6 October 2013. Retrieved 20 September 2013.
The Amended RED incorporated final new safety measures to increase protections for agricultural workers and bystanders. These measures establish a baseline for safe use of the soil fumigants throughout the United States, reducing fumigant exposures and significantly improving safety.
- "Chloropicrin - Frequently Asked Questions". Retrieved 21 October 2013.
- "" Chloropicrin Soil Fumigation in Potato Production Systems"". Plant Management Network. American Phytopathological Society. Retrieved 1 Apr 2019.
- "Control Measures for Chloropicrin" (PDF). California Department of Pesticide Regulation. January 6, 2015. Retrieved January 15, 2015.
added controls for chloropicrin when it is used as a soil fumigant. The controls are intended to reduce risk from acute (short-term) exposures that might occur near fields fumigated with products containing chloropicrin.
- "Chloropicrin Mitigation Proposal" (PDF). Department of Pesticide Regulation - California Environmental Protection Agency. 15 May 2013. p. 2. Archived from the original (PDF) on 6 October 2013. Retrieved 20 September 2013.
The new measures appearing on soil fumigant Phase 2 labels include buffer zones and posting, emergency preparedness and response measures, training for certified applicators supervising applications, Fumigant Management Plans, and notice to State Lead Agencies who wish to be informed of applications in their states.
- Chloropicrin (PS): Lung Damaging Agent. National Institute for Occupational Safety and Health. Emergency Response Safety and Health Database, August 22, 2008. Retrieved December 23, 2008.
- Google Books Library Project – An enhanced card catalog of the world's books. Google. Retrieved 26 January 2015.