Cardenolide
Cardenolide is a type of steroid. Many plants contain derivatives, collectively known as cardenolides, including many in the form of cardenolide glycosides (cardenolides that contain structural groups derived from sugars). Cardenolide glycosides are often toxic; specifically, they are heart-arresting.
 | |
| Names | |
|---|---|
| IUPAC name
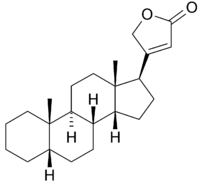
3-[(8R,9S,10S,13S,14R,17S)-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2H-furan-5-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C23H34O2 |
| Molar mass | 342.51486 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Etymology
The term derives from card- "heart" (from Greek καρδία kardiā) and the suffix -enolide, referring to the lactone ring at C17.[1] Cardanolides are a class of steroids (or aglycones if viewed as cardiac glycoside constituents), and cardenolides are a subtype of this class (see MeSH D codes list).
Structure
Cardenolides are C(23)-steroids with methyl groups at C-10 and C-13 and a five-membered lactone (specifically a butenolide) at C-17. They are aglycone constituents of cardiac glycosides and must have at least one double bond in the molecule. The class includes cardadienolides and cardatrienolides. Members include:
- acetyldigitoxins
- acetyldigoxins
- cymarine
- digitoxin
- digitoxigenin
- digoxigenin
- digoxin
- medigoxin
- neoconvalloside
- ouabain
- strophanthins
- strophanthidin
Bufadienolide and Marinobufagenin are similar in structure and function.
Cardanolide is the same core structure but having a saturated lactone ring instead of one containing an alkene.
As defense mechanism
Some plant and animal species use cardenolides as defense mechanisms, notably the milkweed butterflies.[2] Species such as the monarch, queen, and plain tiger ingest the cardenolides contained in the milkweeds (Asclepias) that they mostly feed on and sequester as larvae for defense as adults.[3][4] The cardenolide content in butterflies deters most vertebrate predators, except a few which have evolved to become cardenolide-tolerant, such as the black-backed orioles (Icterus abeillei Lesson) and black-headed grosbeaks (Pheucticus melanocephalus Swainson) that account for 60% of monarch butterfly mortalities in the overwintering sites in central Mexico. In addition to milkweeds and other members of the Apocynaceae, plants in at least 12 botanical families have convergently evolved cardenolides.[5]
References
- Naudé, T. W. (1977). "The occurrence and significance of South African cardiac glycosides". Journal of the South African Biological Society. 18: 7.
- "Interactions with Milkweed | Breeding / Life Cycle | Biology & Natural History | Biology & Research | Monarch Lab".
- Agrawal, Anurag A.; Petschenka, Georg; Bingham, Robin A.; Weber, Marjorie G.; Rasmann, Sergio (April 2012). "Toxic cardenolides: chemical ecology and coevolution of specialized plant-herbivore interactions". The New Phytologist. 194 (1): 28–45. doi:10.1111/j.1469-8137.2011.04049.x. ISSN 1469-8137. PMID 22292897.
- Edgar, J. A.; Cockrum, P. A.; Frahn, J. L. (1976-12-01). "Pyrrolizidine alkaloids inDanaus plexippus L. andDanaus chrysippus L.". Experientia. 32 (12): 1535–1537. doi:10.1007/bf01924437. ISSN 0014-4754.
- Agrawal, Anurag A. (2012). "Toxic cardenolides: chemical ecology and coevolution of specialized plant-herbivore interactions". New Phytologist. 194 (1): 28–45. doi:10.1111/j.1469-8137.2011.04049.x. PMID 22292897.
- "Interactions with Milkweed". Monarch Lab. University of Minnesota. Retrieved 2014-03-25.