We need you! Join our contributor community and become a WikEM editor through our open and transparent promotion process.
Deep brain stimulator complication
From WikEM
Contents
Background
- FDA-approved for medication-refractory movement disorders (e.g. Parkinson's disease, dystonia, essential tremor) and OCD
- Being studied for use in epilepsy, chronic pain, depression, PTSD
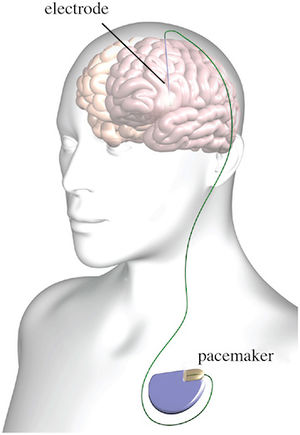
- Single lead systems implanted into the thalamus
- Complication rates range from 7-65%[1]
Clinical Features/Complication Types
- Infection
- Usually early after placement
- Infection rates ~3-10%[2]
- Cerebral hemorrhage
- Lead fracture or migration
- Failure of device to improve tremor/motor symptoms
- Paresthesias (temporary or permanent)
- Dysarthria, decreased verbal fluency
- Disequilibrium
- Affective changes (depression, apathy), impaired executive function, impulsivity
Differential Diagnosis
Evaluation
- May need to observe with device in "off" position to distinguish between device malfunction and other acute neurologic deficit
Management
- Consult neurosurgery
Disposition
See Also
External Links
References
- ↑ Tabbal SD, Revilla FJ, Mink JW, et al. Safety and efficacy of subthalamic nucleus deep brain stimulation performed with limited intraoperative mapping for treatment of Parkinson's disease. Neurosurgery. 2007;61(3 Suppl):119-27.
- ↑ Tabbal SD, Revilla FJ, Mink JW, et al. Safety and efficacy of subthalamic nucleus deep brain stimulation performed with limited intraoperative mapping for treatment of Parkinson's disease. Neurosurgery. 2007;61(3 Suppl):119-27.