Frequently Asked Questions about HPV Vaccine Safety
Featured Resource: HPV Vaccine (Gardasil) is Safe
- What human papillomavirus (HPV) vaccines are available in the United States?
- Are HPV vaccines safe?
- How does CDC make sure HPV vaccines are safe?
- How do the benefits of HPV vaccines compare to the risks?
- Have serious problems been reported after people receive HPV vaccines?
- Can HPV vaccine damage women’s ovaries?
- Has HPV vaccine ever been linked to Guillain-Barré syndrome?
- Can HPV vaccines cause Postural Orthostatic Tachycardia Syndrome (POTS)?
- Has anyone died after receiving HPV vaccines?
- Have FDA and CDC changed any recommendations for the use of HPV vaccines based on vaccine safety monitoring?
- Do HPV vaccines hurt?
- Are HPV vaccines safe for pregnant women?
- Is there anyone who should not get the HPV vaccine?
- What if I have, or my child has, a problem after getting the HPV vaccine?
- Where can I find more information about HPV vaccines?
What HPV vaccines are available in the United States?
There are three HPV vaccines that are licensed for use in the United States by the FDA and recommended by CDC. These vaccines are Gardasil 9, Gardasil, and Cervarix.
| Gardasil 9 | Gardasil | Cervarix | |
|---|---|---|---|
| Who makes it? | Merck & Co., Inc. | Merck & Co., Inc. | GlaxoSmithKline plc |
| What kinds of HPV does it protect against? | HPV types 16, 18, 6, 11, 31, 33, 45, 52, and 58 which cause several types of cancer and genital warts | HPV types 16, 18, 6, and 11, which cause several types of cancer and genital warts | HPV types 16, 18, which cause several types of cancer |
| Who should get the vaccine?* |
|
|
|
|
|||
| When should the vaccine be given? |
|
||
*Recommendations from CDC and the Advisory Committee on Immunization Practices
To find out more about HPV vaccination, please visit www.cdc.gov/HPV.
Are HPV vaccines safe?
Yes. All three HPV vaccines – Gardasil 9, Gardasil, and Cervarix – are safe, effective, and recommended by CDC. Many studies have looked at the safety of HPV vaccines in the United States. An overview of these studies can be found on the vaccine safety publications page.
Vaccines, like any medicine, can have side effects. Some people who get an HPV vaccine have no side effects at all. Some people report having mild side effects, like a sore arm from the shot for a day or two. The most common side effects are usually mild and go away on their own.
Common Side Effects of HPV Vaccine:
- Pain, redness, or swelling in the arm where the shot was given
- Fever
- Headache or feeling tired
- Nausea
- Muscle or joint pain
Fainting (also known as syncope) and related symptoms (such as jerking movements) can happen after any medical procedure. Some people, especially teens, faint after getting vaccinated. To prevent fainting and related injuries, people receiving HPV vaccines should sit or lie down during vaccination, then remain seated for 15 minutes after the shot.
How does CDC make sure HPV vaccines are safe?
All vaccines used in the United States are required to go through years of extensive safety testing before they are licensed by the U.S. Food and Drug Administration (FDA). After vaccines are licensed, CDC and FDA continue to monitor each vaccine to make sure it is safe and effective. CDC’s
The Journey of Your Child's Vaccine explains how all vaccines are developed, tested, and continually monitored.
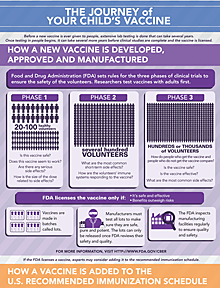
Each HPV vaccine was closely studied in clinical trials to make sure it was safe:
- Gardasil 9 was studied in more than 15,000 females and males.
- Gardasil was studied in 29,000 females and males.
- Cervarix was studied in more than 30,000 females.
These clinical trials showed HPV vaccines to be safe and effective. Each vaccine continues to be monitored for any safety problems. This monitoring is especially looking for any rare or new problems that may happen after vaccination.
CDC uses three systems to monitor the safety of vaccines after they are licensed:
- The Vaccine Adverse Event Reporting System (VAERS)
- The Vaccine Safety Datalink (VSD)
- The Clinical Immunization Safety Assessment (CISA) Network.
Read more about these systems on the Vaccine Safety Monitoring at CDC web page.
How do the benefits of HPV vaccines compare to the risks?
CDC has carefully studied the risks and benefits of HPV vaccination. HPV vaccination is recommended because the benefits, such as prevention of cancer, far outweigh the risks of possible side effects.
Health decisions are personal, and each person should make choices that are right for themselves and their families. It is important to remember that choosing not to vaccinate is not a risk-free choice—HPV vaccines prevent serious cancers and other diseases in both males and females.
Have serious problems been reported after people receive HPV vaccines?
Nearly 90 million doses of HPV vaccines were distributed in the United States from June 2006 through March 2016. Most of CDC’s HPV vaccine safety monitoring and research has focused on Gardasil because this vaccine has accounted for 87% of HPV vaccine doses distributed in the United States. From June 2006 through March 2016, about 79 million doses of Gardasil were distributed in the United States. During the same period, VAERS received 33,945 US reports of adverse events following Gardasil vaccination. There have been 232 US VAERS reports of adverse events following Cervarix (with 720,000 doses distributed) since licensure through March 31, 2016. VAERS has received 1,447 reports of adverse events following Gardasil 9 since licensure through March 31, 2016. About 10 million doses of Gardasil 9 have been distributed in the United States. Any medicine or vaccine can cause adverse events, and (as with any vaccine or medicine) it is difficult to determine whether or not the HPV vaccine caused a particular adverse event.
Which adverse events are considered “serious”?
An adverse event is defined by law as serious if it is life threatening or results in death, a persistent or significant disability or incapacity, congenital anomaly or birth defect, hospitalization, or prolongation of existing hospitalization.
In the VAERS reports, the most frequently reported symptoms overall were: fainting; dizziness; headache; nausea; fever; and pain, redness, and swelling in the arm where the shot was given. Of the reports to VAERS, 7% were classified as “serious.” About 14% of the VAERS reports were not related to health problems, but were reported for reasons such as improper vaccine storage or the vaccine being given to someone for whom it was not recommended.
Can HPV vaccines damage women’s ovaries?
CDC is aware of public concern about the safety of human papillomavirus (HPV) vaccine. Since 2006, HPV vaccine safety monitoring and studies conducted by CDC, FDA and other organizations have confirmed that this vaccine has an excellent safety record without evidence that it causes reproductive problems in women.
What is premature ovarian failure?
Also known as “primary ovarian insufficiency” or “premature menopause,” this is a condition in which a woman’s ovaries stop functioning before age 40. Causes of premature ovarian failure include:
- Genetics
- Chemicals in the environment
- Cancer treatments
- Cigarettes
- Autoimmune disorders
- Some viral infections
However, in many cases it’s impossible to determine the cause. CDC and FDA have not found any proof that Gardasil causes premature ovarian failure.
To read more about HPV and vaccine safety, go to HPV Vaccine Safety.
How has FDA and CDC looked for problems?
Before Gardasil was licensed, its safety was extensively studied in clinical trials. These studies found no difference in amenorrhea (when a woman of reproductive age doesn’t have a period) between women who got Gardasil compared to women who received a placebo (a shot with no medicine in it). Premature ovarian failure (POF) was not found to happen among women in the Gardasil clinical trials.
Between June 2006 and September 2015, more than 80 million doses of Gardasil were distributed for use in the United States. The Vaccine Adverse Event Reporting System (VAERS) received 16 reports of premature ovarian failure and 10 reports of related conditions (ovarian disorder or ovarian failure) being diagnosed at some time after the women received Gardasil vaccination. When adverse events are reported, it does not necessarily mean they are caused by a vaccination. To understand this better, FDA and CDC looked into the reports to see if there was a pattern that might show if the vaccine was causing the problem. There were no patterns among these VAERS reports, so it seems unlikely that the vaccine is causing the problem.
CDC continues to monitor and evaluate the safety of HPV vaccine. Even though VAERS did not see a pattern among reports for POF, the VSD is conducting a study to examine this issue further.
Does HPV vaccine prevent any conditions that lead to loss of a woman’s fertility?
HPV vaccination prevents infection with the HPV types that most commonly cause cervical cancer. In some cases, women develop cervical cancer before starting or finish having children. Treatment for cervical cancer (removal of the cervix and uterus, chemotherapy, and/or radiation) can keep a woman from being able to be pregnant. Preventing cervical cancer through HPV vaccination reduces this risk.
CDC works closely with the American Academy of Pediatrics (AAP) and the American Academy of Family Physicians (AAFP) on HPV vaccination, both of which have information available on their websites:
HPV Vaccine Recommendations [PDF - 660 KB]
AAFP Human Papillomavirus Vaccine
American College of Obstetrics and Gynecologists posts guidelines and recommendations
Has HPV vaccine ever been linked to Guillain-Barré syndrome?
Guillain-Barré syndrome (GBS) is a rare disorder where a person’s own immune system damages nerve cells, causing muscle weakness and sometimes paralysis. Most people recover fully from GBS, but some experience long-term nerve damage.
CDC’s Vaccine Safety Datalink conducted monitoring for GBS following Gardasil vaccination from August 2006 to February 2012. During this period just over 1.4 million doses of Gardasil were administered in the Vaccine Safety Datalink population. CDC did not identify any cases of GBS among females aged 9-26 years old following Gardasil vaccination.
Can HPV vaccines cause Postural Orthostatic Tachycardia Syndrome (POTS)?
POTS is a condition that causes lightheadedness or fainting and a rapid increase in heartbeat upon standing. The cause is unknown, but doctors think POTS may be associated with a number of risk factors and syndromes, including: a recent viral illness, physical deconditioning, chronic fatigue syndrome and nervous system problems. In November 2015, the European Medicine’s Agency completed a detailed review of available POTS data [PDF - 72 KB] from young women who received HPV vaccines. The review found that the evidence does not support a causal link between HPV vaccines and POTS.
From June 2006 through September 2015, when about 80 million doses of Gardasil doses have been given out in the United States, VAERS have received 13 reports of POTS following 4vHPV. There are no unusual or unexpected patterns detected among these cases of POTS following Gardasil vaccination. CDC monitoring through VAERS has not detected any safety concern of POTS following HPV vaccination.
Has anyone died after receiving an HPV vaccine?
Some deaths among people who received an HPV vaccine have been reported to VAERS. This does not mean that the vaccine caused the death, only that the death occurred after the person got the vaccine. CDC and FDA review all available information on reports of death following all vaccines, including HPV vaccines.
From June 2006 through September 2015, when about 80 million doses of HPV vaccine had been given out in the United States, VAERS received 117 reports of death after people received the Gardasil vaccine. Among the 117 reports of death, many could not be further studied because there was not enough information included in the report to verify that a person had died. In 51 of the reports, CDC reviewed medical records, autopsy reports, or death certificates and verified that the person had died. After careful review of every reported case of death that has happened after Gardasil vaccination, CDC concluded:
- There is no diagnosis that would suggest Gardasil caused the death
- There is no pattern of death occurring with respect to time after vaccination
- There is no consistent vaccine dose number or combination of vaccines given among the reports
Have FDA and CDC changed any HPV vaccine recommendations based on vaccine safety monitoring?
Yes. When fainting (or syncope) was found to happen after vaccination, FDA changed Gardasil’s guidance for doctors to include information about preventing falls and injuries from fainting after HPV vaccination. CDC and the Advisory Committee on Immunization Practices included this guidance in the recommendations for HPV vaccination. CDC continues to remind doctors and nurses to observe this guidance and to share this information with all their patients.
Do HPV vaccines hurt?
Some people who get the HPV vaccine may have some pain in the arm where the shot was given. Usually this pain is mild and goes away within a few days. Swelling and redness also sometimes occur after HPV vaccination.
CDC is aware of reports (in Japan and elsewhere) of chronic pain following HPV vaccines. Some of these reports might be potential cases of Complex Regional Pain Syndrome (CRPS), a rare condition of persistent pain that usually affects arms, legs, hands or feet after an injury or trauma to that limb. In November 2015, the European Medicine’s Agency completed a detailed review of available data on CRPS [PDF - 72 KB] in young women who received HPV vaccines. The review found that the evidence does not support a causal link between HPV vaccines and CRPS.
A published article found 21 reports of CRPS to VAERS following to Gardasil between June 2006 and July 2015 concluding that CRPS following Gardasil is rare. CDC monitoring through VAERS has not detected any safety concern for CRPS following HPV vaccination.
Are HPV vaccines safe for pregnant women?
Because the vaccines weren’t tested in pregnant women during clinical trials, HPV vaccines are not currently recommended for pregnant women. However, some pregnant women receive HPV vaccines because they don’t know that they are pregnant at the time of vaccination.
CDC and vaccine manufacturers have monitored and studied HPV vaccine safety in women who received the vaccine when they were pregnant. Close monitoring has not found any health concerns. If a woman receives HPV vaccine and later learns that she is pregnant, there is no reason to be alarmed.
Any woman who learns she was pregnant at the time she received an HPV vaccine is encouraged to contact the vaccine manufacturer. This will help us learn how pregnant women respond to the vaccine.
- Pregnant women who received Gardasil 9 or Gardasil can contact Merck at 1-877-888-4231 if they have questions related to getting the vaccine while pregnant.
- Doctors should report Gardasil 9 vaccination during pregnancy as early in the pregnancy as possible using Merck Pregnancy Registries.
- Pregnant women who received Cervarix can contact GlaxoSmithKline at 1-888-452-9622
Is there anyone who should not get an HPV vaccine?
Some people should not get the HPV vaccine or should wait.
- Anyone who has ever had a life-threatening allergic reaction to any component of HPV vaccine, or to a previous dose of HPV vaccine, should not get the vaccine.
- If you or your child is considering receiving Gardasil 9 or Gardasil, tell your doctor about any severe allergies, including an allergy to yeast.
- If you or your child is considering receiving Cervarix, tell your doctor about any severe allergies, including an allergy to latex.
- HPV vaccine is not recommended for pregnant women. However, receiving HPV vaccine when pregnant is not cause for alarm. Women who are breastfeeding may get the vaccine.
- People who are mildly ill (low-grade fever of less than 101 degrees, a cold, runny nose, or cough) when a dose of HPV vaccine is planned can still be vaccinated. People with a moderate or severe illness should wait until they are better.
What if I have, or my child has, a problem after getting an HPV vaccine?
If you or your child is having a severe allergic reaction or other health emergency, call 9-1-1 or go to the nearest hospital.
Look for any signs or symptoms that concern you, such as signs of a severe allergic reaction, very high fever, or behavior changes. Signs of a severe allergic reaction can include hives, swelling of the face and throat, difficulty breathing, a fast heartbeat, dizziness, and weakness. These would start a few minutes to a few hours after the shot is given.
After seeing a doctor, the reaction should be reported to the Vaccine Adverse Event Reporting System (VAERS). This system is used to report any side effect or adverse event following vaccination. Your doctor can file this report, or you can do it yourself through the VAERS website or by calling 1-800-822-7967.
Where can I find more information about HPV vaccines?
For additional information on HPV vaccines and CDC’s recommendations, please visit CDC's Human Papillomavirus (HPV) website.
- Page last reviewed: November 2, 2015
- Page last updated: January 23, 2017
- Content source:


 ShareCompartir
ShareCompartir
