Vaccine Safety Datalink (VSD)
The Vaccine Safety Datalink (VSD) is a collaborative project between CDC’s Immunization Safety Office and eight health care organizations. The VSD started in 1990 and continues today in order to monitor safety of vaccines and conduct studies about rare and serious adverse events following immunization.
The VSD uses electronic health data from each participating site. This includes information on vaccines: the kind of vaccine given to each patient, date of vaccination, and other vaccinations given on the same day. The VSD also uses information on medical illnesses that have been diagnosed at doctors’ offices, urgent care visits, emergency department visits, and hospital stays. The VSD conducts vaccine safety studies based on questions or concerns raised from the medical literature and reports to the Vaccine Adverse Event Reporting System (VAERS). When there are new vaccines that have been recommended for use in the United States or if there are changes in how a vaccine is recommended, the VSD will monitor the safety of these vaccines.
The VSD has a long history of monitoring and evaluating the safety of vaccines. Since 1990, investigators from the VSD have published many studies to address vaccine safety concerns. Examples of VSD’s work include the following:
- White Paper on Studying the Safety of the Childhood Immunization Schedule [PDF – 896K]
- Are vaccines that contain additives safe for children? How about vaccines with preservatives?
- Are rotavirus vaccines safe for infants?
- Do vaccines cause febrile seizures?
- Are there safety concerns following HPV vaccine?
Participating VSD Healthcare Organizations
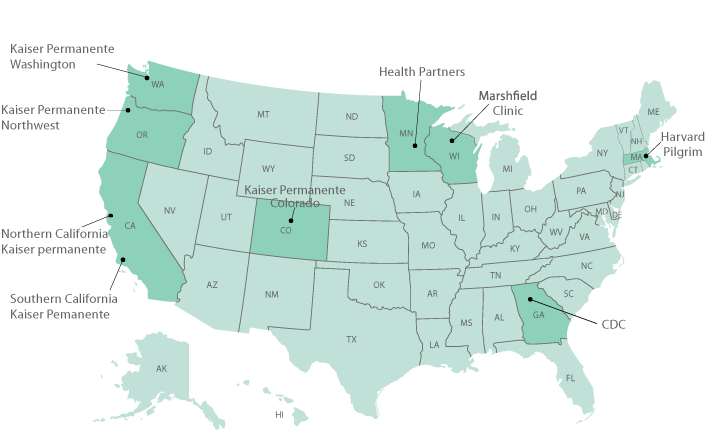
- Group Health Cooperative of Puget Sound, Seattle, Washington
- Harvard Pilgrim Health Care, Boston, Massachusetts
- HealthPartners Research Foundation, Minneapolis, Minnesota
- Kaiser Permanente Northwest, Portland, Oregon
- Kaiser Permanente Medical Care Program of Northern California, Oakland, California
- Kaiser Permanente Colorado, Denver, Colorado
- Kaiser Permanente of Georgia, Atlanta, GA
- Marshfield Clinic Research Foundation, Marshfield, Wisconsin
- Southern California Kaiser Permanente Health Care Program, Los Angeles, California
Objectives of the VSD
- To conduct research on important vaccine safety questions in large populations
- To conduct vaccine safety studies that come from questions or concerns in the medical literature or from other vaccine safety systems, like VAERS
- To monitor possible adverse events when new vaccines are licensed or when there are new vaccine recommendations
- To provide information to committees who make recommendations for the nation
Rapid Cycle Analysis
Rapid Cycle Analysis (RCA) allows VSD to detect adverse events following vaccination in near real time so the public can be informed quickly of possible risks.
Using VSD data that are updated each week, the rates of adverse events that occur in people who have received a particular vaccine are compared to the rate of adverse events that occurs in a similar group of people who have not received that vaccine. If the rate of adverse events among vaccinated people is higher than among the comparison group, the vaccine may be associated with an adverse event. VSD has used RCA to publish important safety information regarding many vaccines, including:
- Pentacel (DTaP-IPV/Hib) (diphtheria, tetanus, pertussis [DTap], HiB, and polio)
- Kinrix (DTaP-IPV) (DTaP and polio)
- Gardasil (human papillomavirus)
- Influenza
- Rotavirus
- Meningococcal
- Measles, mumps, rubella, and varicella (MMRV)
- Tetanus, Diphtheria, Pertussis (Tdap)
Evaluating Safety of Vaccines in Pregnancy
Vaccination protects pregnant women and unborn babies from several preventable diseases. Because it is so important to protect pregnant women, evaluating the safety of vaccines given to women during pregnancy [PDF – 181 KB] is a high priority for the VSD.
Using an electronic health record to determine a woman’s stage in her pregnancy is complicated. The VSD has developed algorithms to identify pregnant women and determine the start and end dates of the pregnancy. VSD is also able to use data to study the health of children born to women who were vaccinated during pregnancy. VSD has published 14 studies related to pregnancy and vaccination during pregnancy and is currently studying the safety of Tdap vaccination, inadvertent HPV vaccination, and influenza vaccination during pregnancy.
Research Methods to Study Vaccine Safety
VSD investigators have pioneered the development of statistical methods to conduct valid, accurate vaccine safety studies. Methods developed by the VSD in the past have been adopted by other researchers using large-linked databases and VSD investigators continue to develop and refine statistical approaches for vaccine safety research. Methods developed by the VSD include:
- maximized sequential probability ratio test (MaxSPRT [PDF- 259 KB]) (CMaxSPRT)
- group sequential analysis
- case-centered analysis
These methods allow VSD to conduct timely and reliable vaccine safety monitoring and evaluations.
How Can I Access VSD Data?
From time to time, scientists (from outside CDC and outside the VSD network) are interested in using data from the VSD to look differently at vaccine safety questions. When possible, CDC tries to accommodate these requests. Depending on the study, interested researchers may be able to access VSD data and data from VSD publications through public use datasets, the VSD data sharing program, and collaboration with current VSD investigators.
- Page last reviewed: August 30, 2017
- Page last updated: September 8, 2017
- Content source:


 ShareCompartir
ShareCompartir